María Laura Fernández-Salinas a, *, Marcia Luciana Matoz-Fernández b
a Universidad Nacional de Jujuy, Instituto de Biología de la Altura, Avenida Bolivia 1661, 4600 San Salvador de Jujuy, Jujuy, Argentina
b Universidad Nacional de Mar del Plata, Laboratorio de Zoonosis Parasitarias, Funes 3350, 7600 Mar del Plata, Buenos Aires, Argentina
*Corresponding author: mfernandez@inbial.unju.edu.ar (M.L. Fernández-Salinas)
Received: 31 January 2024; accepted: 10 June 2024
Abstract
The genus Myianoetus Oudemans(Acari: Histiostomatidae) is commonly associated with carrion, utilizing flies (Diptera) from various families as a means of dispersal through phoresy. The objective of this paper is to present a new association between Myianoetus sp. mites and Calliphoridae flies and discuss its relevance in forensic sciences. Samples were collected in 3 locations in the Prepuna ecoregion of Jujuy, Argentina. Specimens were captured using necrotraps baited with cow lung. Flies carrying phoretic mites were separated and identified to a specific level, while mites were counted and identified at the lowest possible taxonomic level. Compsomyiops fulvicrura (Robineau-Desvoidy) (Diptera: Calliphoridae) was the only species that presented attached mites, with an average intensity of 12.26 mites per fly. The mites carried by C. fulvicrura were identified as deutonymphs of Myianoetus sp., with a prevalence of 2.56% of infested flies. Significant differences in the abundance of flies with mites were observed between locations and seasons. This article represents the first contribution to knowledge on the specific association between Myianoetus sp. and C. fulvicrura. These findings in forensic ecology are relevant for their potential contribution and application in the development of more precise methods in specific forensic cases.
Keywords: Astigmata; Diptera; Forensic Acarology; Phoresy; New report
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Asociación de Myianoetus sp. (Acari: Histiostomatidae) con la mosca necrófaga Compsomyiops fulvicrura (Diptera: Calliphoridae), en la ecoregión Prepuna (Jujuy: Argentina)
Resumen
El género Myianoetus Oudemans (Acari: Histiostomatidae) suele asociarse a la carroña utilizando moscas (Diptera) de distintas familias como medio de dispersión, a través de la foresia. El objetivo de este trabajo fue presentar una nueva asociación entre Myianoetus sp. con moscas Calliphoridae y discutir su alcance dentro de las ciencias forenses. Las muestras se recolectaron en 3 localidades de la Prepuna jujeña, Jujuy, Argentina. Los especímenes se capturaron mediante necrotrampas cebadas con pulmón vacuno. Las moscas con ácaros se separaron y determinaron a nivel específico; los ácaros fueron numerados e identificados al nivel taxonómico más bajo posible. Compsomyiops fulvicrura (Diptera: Calliphoridae) fue la única especie que presentó ácaros adheridos, con una intensidad media de 12.26 ácaros por mosca. Los ácaros fueron identificados como deutoninfas de Myianoetus sp. y se determinó una prevalencia de 2.56% de moscas infestadas. Se observaron diferencias significativas en la abundancia de moscas con ácaros entre las localidades y estaciones analizadas. Este artículo representa el primer aporte al conocimiento sobre la asociación específica entre Myianoetus sp. y C. fulvicrura. Estos hallazgos sobre ecología forense son relevantes por su potencial contribución y aplicación al desarrollo de métodos más precisos en casos forenses determinados.
Palabras clave:Astigmata; Diptera; Acarología forense; Foresia; Nuevo reporte
Introduction
Carcasses present limited and ephemeral biocenosis made up of diverse organisms that often comprise complex food webs (Braig & Perotti, 2009; Perotti et al., 2010). Many Diptera species actively participate in the cadaveric decomposition process in which the Calliphoridae and Sarcophagidae families, along with Coleoptera are often investigated because of their large number, persistence and capacity to act as hosts to diverse mites that use them for dispersion by phoresis (Camerick, 2010; Perotti & Braig, 2009; Perotti et al., 2010).
Mites present morphological and physiological adaptations to serve phoresy during adult and nymphal stages. These adaptations are documented in the order Mesostigmata, in the suborder Prostigmata and in the infraorder Astigmatina (Oribatida) (Perotti et al., 2010). Astigmata mites are specialists in irregular or ephemeral habitats which they colonize through a deutonymphal heteromorphic stage known as hypopus which is specialized for phoresy (OConnor, 2009). Astigmatid deutonymphs are morphologically simplified, have lost the mouth and chelicerae, have greatly reduced the remainder of the gnathosoma, and have suckers on the paraproctal region for efficient phoretic attachment. The body is strongly dorsoventrally flattened, heavily sclerotized and much more resistant to desiccation than other stages of the life cycle (Farish & Axtell, 1971; OConnor, 1982). The conditions needed to reach this stage may involve genetic factors and physicochemical factors from the environment (Greenberg & Carpenter, 1960).
Astigmatid mites are particularly important for the 3 areas of forensic entomology: urban, stored product pests and medico-legal (Catts & Goff, 1992; Perotti & Braig, 2019). Nevertheless, they often go unnoticed because of their small size. Moreover, their analysis is limited because of difficulties in species identification, lack of specific knowledge and misuse of forensic methodology (OConnor, 2009; Perotti et al., 2010). Numerous species of mites are compulsory or facultative inhabitants of carrion. They are found not only in legal cases that involve human carcasses (Pimsler et al., 2016; Rai et al., 2020; Russell et al., 2004; Saloña-Bordas & Perotti, 2015); but also, in experimental studies concerning faunal succession in animal remains (Arnaldos et al., 2005; Barton et al., 2014; Centeno & Perotti, 1999; Heo et al., 2021).
In Argentina, the only record of the presence of phoretic mites associated with decomposing remains were the preliminary observations of Centeno and Perotti (1999), in which they found mites of the genus Myianoetus Oudemans (Astigmata: Histiostomatidae) associated with a specimen of Morellia sp. (Muscidae). In order to contribute to the further study on phoretic relations between mites and arthropods, this paper presents a new association between mites and Diptera from the Calliphoridae family in Prepuna of Jujuy, Argentina, and discusses its relevance and importance within the forensic sciences.
Materials and methods
The collection of Diptera specimens was carried out in the following locations: Tres Cruces (22°55’06.01” S, 65°35’13.58” W), Humahuaca (23°12’14.27” S, 65°20’54.90” W), and Tumbaya (23°51’27.79” S, 68°28’03.31” W) (Fig. 1a-c). These locations are part of the Monte Province, Prepuna District in the province of Jujuy, Argentina. Two sampling campaigns were carried out, one during the dry season (June, July, and August) and the other during the wet season (December, January, and February) between 2016 and 2018. Each location was equipped with 18 traps, totaling 54 traps across all sampling locations, totaling 108 traps per year.
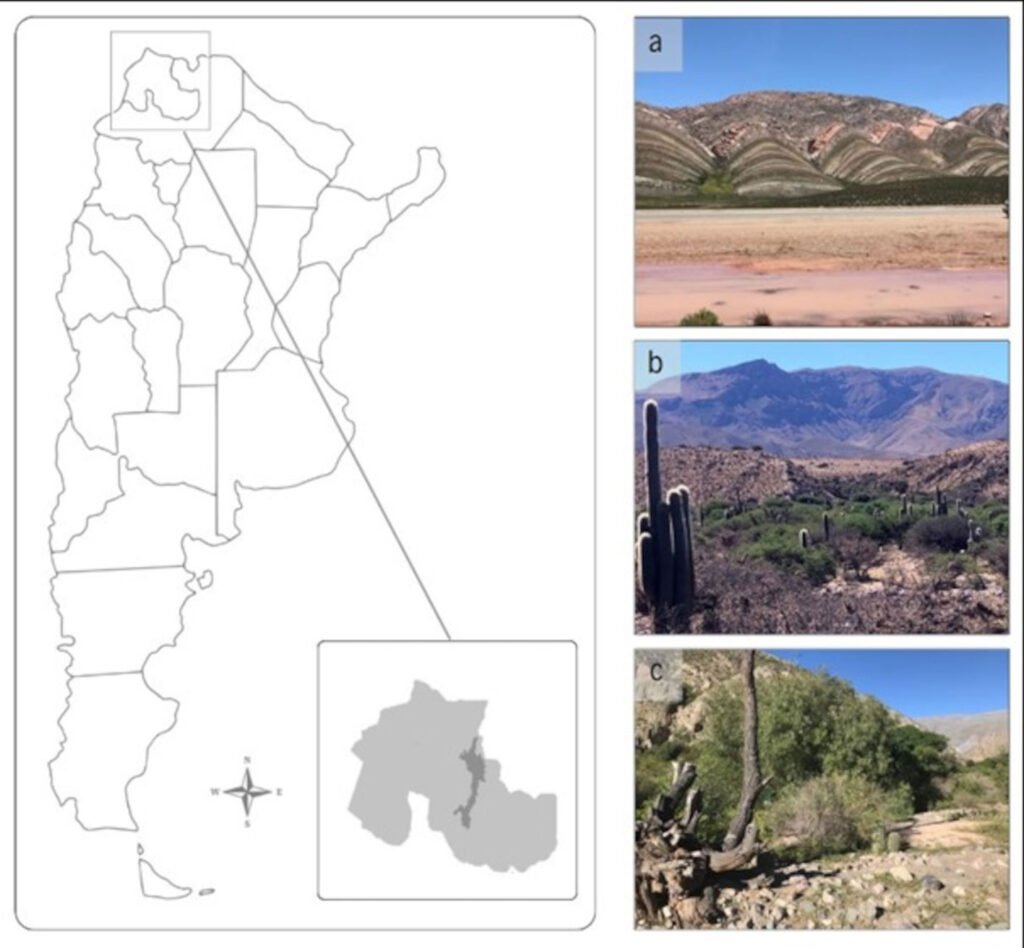
Figure 1. Location of the study area. The map depicts the region corresponding to the Monte Province, Prepuna District, in the province of Jujuy, Argentina. Study sites are located in the following localities: a) Tres Cruces, b) Humahuaca, and c) Tumbaya (Photos by Fernández Salinas, M. L.).
To obtain specimens in good condition for identification, traps were made following Hwang and Turner (2005) (Fig. 2). A modified cone trap, based on a soft drink bottle with a baited target, was constructed. The bottle traps were assembled using two 3 L clear plastic soft drink bottles with a diameter of 11.5 cm, along with a black acrylic container 11 cm measured in diameter and 13 cm in depth. Consisted of 2 parts, the upper collection chamber and the lower bait chamber. The collection chamber was formed from the bottles cut 24 cm and 12 cm from the top respectively, one pushed inside the other (so that the bottle’s spout acts as a funnel and prevents flies from escaping). The bait chamber was made with a black container so the flies were drawn upwards, into the transparent collection chamber. The 2 halves of the trap were push-fitted together and secured by strips of waterproof adhesive tape. To facilitate the entry of flies 4 holes of approximately 0.8 cm in diameter were made around the bait chamber. A 125 cm³ plastic container with the bait was placed at the base of this container. A feeding substrate made of 100 g of cow’s lung was used. A distance of approximately 100 m was maintained between the traps because of the competitive nature of the colonizing species and were separated from the floor as they were hung at a minimum height of 1.5 m to avoid the attack of scavenger mammals. It was placed in a closed recipient which was subjected to a warm temperature between 15 °C and 30 °C, during 60 hours, for sufficient time to decompose. The traps were left in place for 7 consecutive days.
The captured specimens were put in Kahn tubes with 70% alcohol and they were transported to the Institute of Altitude Biology (INBIAL), San Salvador de Jujuy, Jujuy, Argentina.
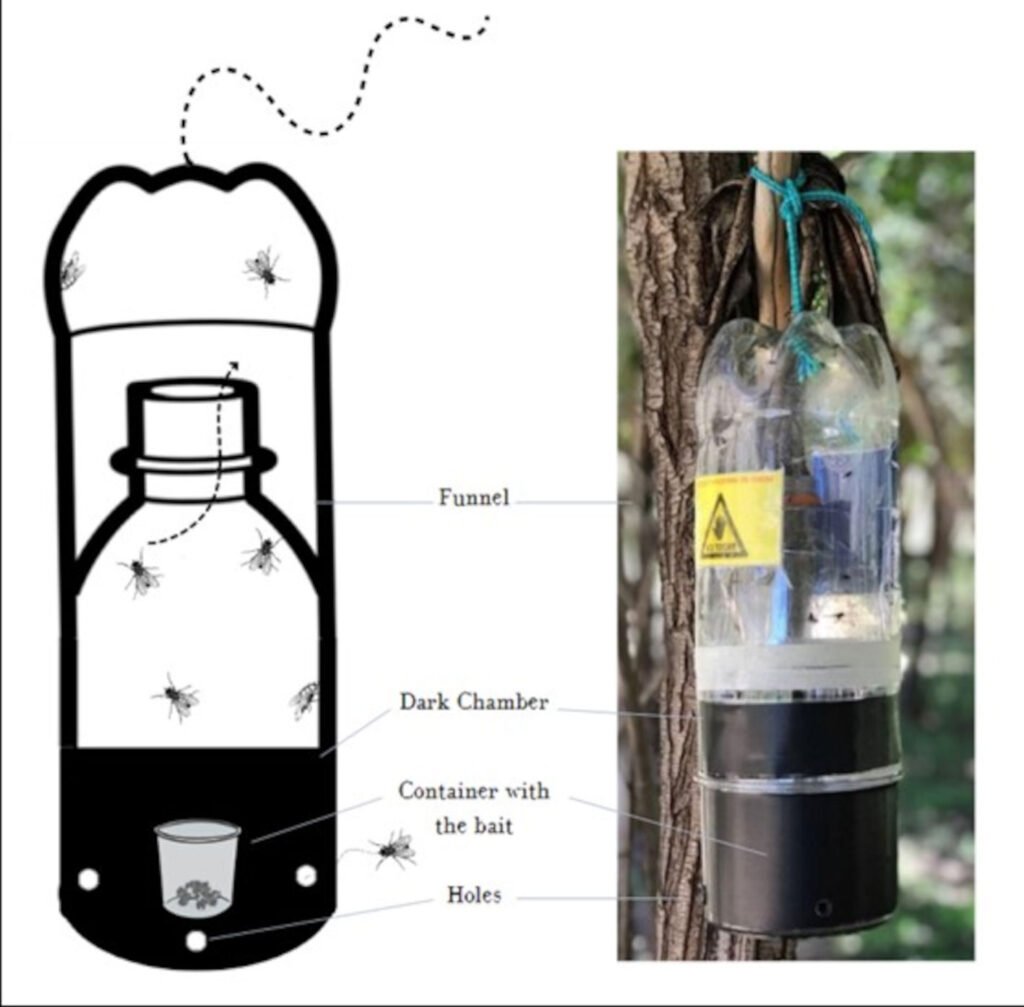
Figure 2. Design of the bottle necrotrap baited with cow lung.
Flies that presented mites were counted, separated by sex and identified to its most specific level using keys and revisions from Olea and Mariluis (2013), Whitworth (2014), and Mulieri et al. (2014). The flies were photographed “in situ” using a Canon 5D Mark IV camera, 3 extension tubes for macro photography and a Canon 85 mm 1.8 lens illuminated with a Godox AD200 flash and a Godox V860 flash. Afterward, the specimens were sent to the Parasitic Zoonoses Laboratory, National University of Mar del Plata, Mar del Plata, Buenos Aires, Argentina. Each fly was individually examined, and the number of mites per fly and their attachment sites on the host flies were determined. The mites were then removed with the assistance of fine-tipped needles. From selected mite specimens, permanent preparations in Hoyer’s medium were made. The remaining specimens were identified from temporary preparations after being cleared in lactic acid using an open slide technique in order to be observed under the optic microscope (Olympus CX31). Taxonomic identification was done at a genus level using diagnostic keys (Dindal, 1990). Mites were photographed with a Sony Powershot DSC-P200 camera. The photographs were edited with Adobe Photoshop CS.
Table 1
Percentage of prevalence and mean intensity of Myianoetus sp. associated with Compsomyiops fulvicrura, in 3 locations of Jujuy, Argentina.
| Location | Season | Nº of flies with attached mites | Total Nº of flies | Nº of mites | Prevalence (%) | Mean Intensity |
| Tumbaya | Dry | 26 | 661 | 243 | 3.93 | 9.34 |
| Wet | 0 | 85 | 0 | 0 | 0 | |
| Humahuaca | Dry | 40 | 1,517 | 478 | 2.63 | 11.95 |
| Wet | 0 | 205 | 0 | 0 | 0 | |
| Tres Cruces | Dry | 18 | 624 | 156 | 2.88 | 8.83 |
| Wet | 10 | 582 | 276 | 1.71 | 27.3 |
The abundance of flies with attached mites was analyzed using Generalized Linear Models (GLM) through the software InfoStat (Di Rienzo et al., 2020). In the model, the 3 study locations were considered as fixed effects while the seasons were treated as random variables. Variance heterogeneity was adjusted using the VarExp variance function, and models were selected according to Akaike (AIC) and Bayesian (BIC) criteria. Subsequently, a Fisher’s LSD test (α = 0.05) of adjusted means and standard errors was conducted to evaluate differences between locations, following the methods described. Prevalence and mean intensity were calculated as indicated by Bush et al. (1997) and Margolis et al. (1982). Prevalence was calculated as the number of flies infected with phoretic mites, divided by the number of flies examined in a sample, and was expressed as a percentage. The mean intensity of phoresy was defined as the total number of phoretic mites of a particular species found in a sample, divided by the number of host flies.
For further taxonomic studies, voucher species were deposited as slide-mounted specimens in the Entomological Collection “Dr. Lilia Estela Neder”, Institute of Altitude Biology (INBIAL), National University of Jujuy, Jujuy, Argentina (INBIAL C 15000; INBIAL C 15001).
Results
A total of 9,454 Calliphoridae individuals were collected. They spanned 5 genera and 12 species: Calli-
phora vicina (Robineau-Desvoidy), Chlorobrachycoma versicolor (Bigot), Chrysomya albiceps (Wiedemann), Chrysomya megacephala (Fabricius), Cochliomyia mace-llaria (Fabricius), Cochliomya hominiborax (Coquerel), Compsomyiops fulvicrura (Robineau-Desvoidy), Com-psomyiops sp., Lucilia cuprina (Wiedemann), Lucilia sericata (Meigen), Sarconesia chlorogaster (Wiedemann), Sarconesiopsis magellanica (Le Guillou). The most abundant species were C. albiceps and C. fulvicrura with 4,651and 3,674 individuals respectively. C. fulvicrura was the only species that had mites attached to its body (Fig. 3). These mites primarily attached themselves to the thorax and head regions and were identified as deutonymphs of Myianoetus sp. (Figs. 4, 5, 6). The individuals found exhibit morphological similarities to the deutonymphs of Myianoetus muscarum (Linnaeus) (OConnor et al., 2015). However, they differ from this species by possessing dorsal hysterosomal setae of approximately equal length to the exobothridial setae, unlike M. muscarum, where the hysterosomal setae are less than half the length of the exobothridial setae. Given that this characteristic is diagnostic of M. muscarum, we hypothesize that the specimens uncovered in this study may represent a yet undescribed species. Out of the total number of C. fulvicrura individuals, 94 carried phoretic mites (83 females and 11 males), representing a prevalence of 2.56% (Table 1). A total of 1,153 mites were counted, which corresponds to a mean intensity of 12.26 mites/fly (1-89 rank) (Fig. 7). The majority of mites (76%) were found during the dry season in all 3 studied locations. However, in Tres Cruces, mites were also found during the wet season (Table 1).
Table 2
Summary of generalized linear model (GLM) analysis results and model fitting parameters. Significance levels (p values) and variance function parameters, model fitting parameters including number of observations (N), Akaike information criterion (AIC), Bayesian information criterion (BIC), the log probability, the standard deviation (Sigma) and the coefficient of determination (R2) are shown.
| Effects | p-value | Variance function parameters |
| Location | < 0.0001 ** | |
| Season | 0.0105 * | -0.27 (dry) |
| -0.09 (wet) |
Model tuning: N = 6, AIC = 31.83, BIC = 22.68, LogLik = -8.91, Sigma = 4.82, R2 = 0.74
Table 3
Results of the Fisher’s LSD test (α = 0.05): adjusted means and standard errors for the 3 locations under study. Common letters indicate that the means do not differ significantly (p > 0.05).
| Location | Means | SE | |
| Humahuaca | 20.84 | 1.98 | A |
| Tumbaya | 6.84 | 1.98 | B |
| Tres Cruces | 1.16 | 1.98 | C |
The GLM analysis revealed significant differences in the abundance of flies with attached mites among the study locations (p < 0.0001) and a significant effect of seasonality (p = 0.0105) (Table 2).

Figure 3. Deutonymphs of Myianoetus sp. (yellow arrow) between the thorax and abdomen of Compsomyiops fulvicrura.
Additionally, subsequent Fisher’s LSD analysis revealed statistically different groups among the study locations. A higher mean abundance of flies with attached mites was observed in Humahuaca, followed by Tumbaya and Tres Cruces (Table 3). It is noteworthy that the highest variance parameter for the dry season (-0.27) compared to the wet season (-0.09) suggests that these differences are primarily attributed to this time of the year, between June and August.

Discussion
The genus Myianoetus comprises more than 40 species widespread throughout the world (OConnor et al., 2015), most known only from deutonymphs phoretic on Diptera. In this work, the association between deutonymphs of Myianoetus sp. with C. fulvicrura is described for the first time. Up to present, there are reports of deutonymphs from the Myianoetus that have been found associated with various Diptera families: Sphaeroceridae (Fain et al., 1980), Muscidae (Centeno & Perotti, 1999; Greenberg & Carpenter 1960; Negm & Alatawi, 2011; Pimsler et al., 2016), Calliphoridae (Greenberg & Carpenter 1960; Miranda & Bermúdez, 2008) and Heleomyzidae (Zamec & Košel, 2014). Evidence obtained from lab experiments further described the phoretic interaction of the hypopi of Myianoetus muscarum with Muscina stabulans Fallen (Diptera: Muscidae), Stomoxys calcitrans Linnaeus (Diptera: Muscidae), Lucilia sericata (Diptera: Calliphoridae) and Musca domestica Linnaeus (Diptera: Muscidae) (Greenberg & Carpenter, 1960). Additionally, in a study carried out in Texas, USA, by Pimsler et al. (2016), a great number of M. muscarum individuals associated with Synthesiomyia nudiseta (Wulp) (Diptera: Muscidae) were collected in 3 human corpses.
Among the Calliphoridae species collected, C. albiceps stood out as the most abundant. However, deutonymphs of Myianoetus sp. were exclusively phoretically associated with C. fulvicrura. The statistical differences observed in the abundances of flies with attached mites among the different studied locations suggest that these were influenced by the dry season. Therefore, the preference for C. fulvicrura could be associated with seasonal variation, as it was more abundant during the dry season, contrasting with C. albiceps, which showed a preference for the wet season. These trends were notable in Tumbaya and Humahuaca, where C. albiceps was the dominant species, while in Tres Cruces, the abundance of this species was very low, with C. fulvicrura being the dominant species in both seasons in that area. Additionally, it is plausible that this choice is related to the chemical attraction of mites to volatile substances released by the puparia of C. fulvicrura, as demonstrated in the studies by Greenberg and Carpenter (1960). These observations were reflected in the prevalence values, which indicated higher values during the dry season in all 3 locations, compared to the wet season.
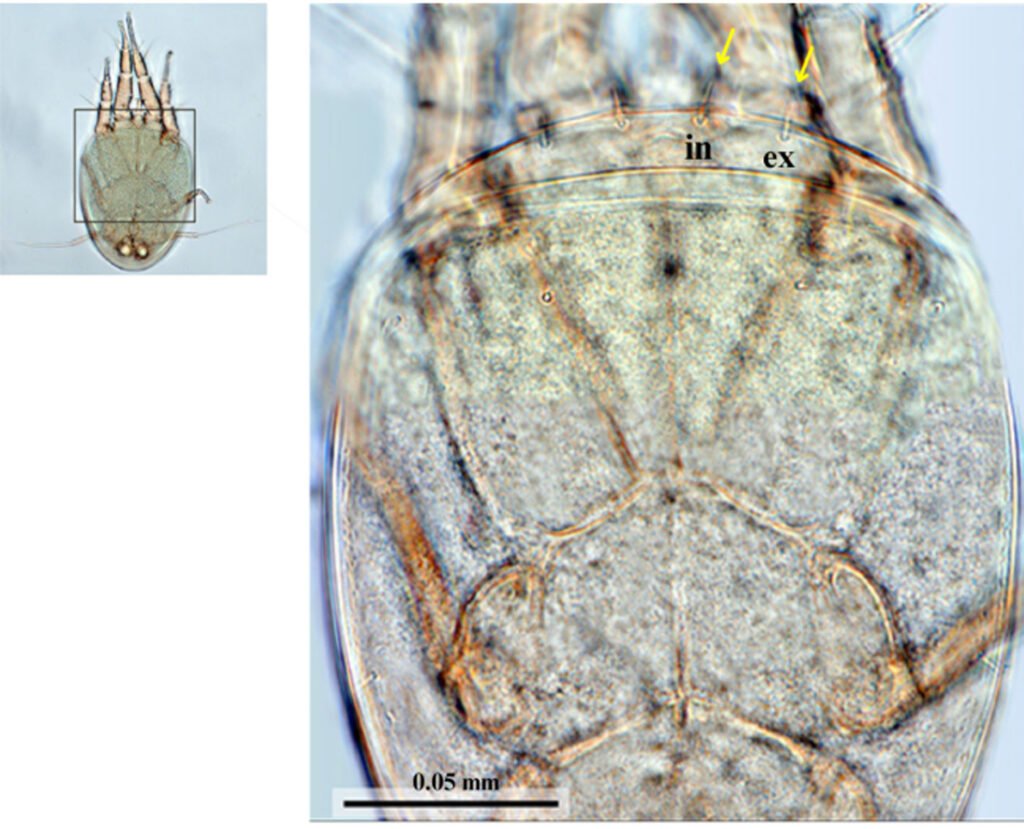
Figure 5. Dorsal view of Myianoetus sp. (scale = 0.05 mm). The yellow arrow indicates the hysterosomal setae (ex) and exobothridial setae (in).

Figure 6. Dorsal view of legs I and II of Myianoetus sp. (scale = 0.1 mm). The yellow arrow shows the bifurcate empodial claw, characteristic of the genus, present on legs I-III.

Figure 7. Abundance frequency (AF) histogram of Myianoetus sp. deutonymphs associated with Compsomyiops fulvicrura individuals.
The lack of interaction between Myianoetus sp. with other species and its demonstrated affinity with C. fulvicrura suggest that these mites can be phoretically selective in the Prepuna environment. At genus or species level, mites have micro-habitat specific requirements, being excellent specific environmental indicators, offering themselves as potentially one of the most informative pieces of biological trace evidence collected from a crime scene (Perotti & Braig, 2019). This may explain events of corpse location, of relocation, a link to a suspect and a possible connection between a suspect and a victim or a crime scene (Hani et al., 2018; Kamaruzaman et al., 2018; Szeleczl et al., 2018). The specificity and abundance of mites, coupled with the intensity of phoresy, could contribute to estimating more precise post-mortem intervals (PMI) (Miranda & Bermúdez, 2008; Rodrigueiro & Prado, 2004; Russell et al., 2004). In addition, Perotti and Braig (2009) suggested that the presence of a specific phoretic mite (for example Myianoetus sp.) may confirm the presence of its host (for example C. fulvicrura), even when the host is already gone.
Given that mites are a valuable forensic tool, it is crucial to deepen the understanding of the biology and ecology of the species involved. To expand this knowledge, it is necessary to continue registering and investigating new species and their phoretic associations under various climatic and biogeographical conditions.
Acknowledgements
We would like to thank Pablo A. Martínez for his critical analysis and recommendations for our manuscript, Mario A. Linares for the Myianoetus sp.photograph and Ismael Acosta for the C. fulvicrura photograph.
References
Arnaldos, M. I., García, M. D., Romera, E., Presa, J. J., & Luna, A. (2005). Estimation of post-mortem interval in real cases based on experimentally obtained entomological evidence. Forensic Science International, 149, 57–65. https://doi.org/10.1016/j.forsciint.2004.04.087
Barton, P. S., Weaver, H. J., & Manning, A. D. (2014). Contrasting diversity dynamics of phoretic mites and beetles associated with vertebrate carrion. Experimental and Applied Acarology, 63, 1–13. https://doi.org/10.1007/s10493-013-9758-7
Braig, H. R., & Perotti, M. A. (2009). Carcasses and mites. Experimental and Applied Acarology, 49, 45–84. https://doi.org/10.1007/s10493-009-9287-6
Bush, A. O., Lafferty, K. D., Lotz, J. M., & Shostak, A. W. (1997). Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. The Journal of Parasitology, 83, 575–583. https://doi.org/10.2307/3284227
Camerik, A. M. (2010). Phoresy revisited. In M. Sabelis, & J. Bruin (Eds.), Trends in Acarology (pp. 333–336). Dordrecht: Springer. https://doi.org/10.1007/978-90-481-9837-5_53
Catts, E. P., & Goff, M. L. (1992). Forensic entomology in criminal investigations. Annual Review of Entomology, 37, 253–272. https://doi.org/10.1146/annurev.en.37.010192.001345
Centeno, N. D., & Perotti, M. A. (1999). Ácaros vinculados a procesos de descomposición de cadáveres y sus posibles asociaciones foréticas. In Actas y Trabajos de la XIX Reunión Argentina de Ecología, 1999. Tucumán, Argentina.
Di Rienzo J. A., Casanoves, F., Balzarini, M. G., González, L., Tablada, M., & Robledo, C. W. (2020). InfoStat versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
Dindal, D. L. (1990). Soil biology guide. New York: John Wiley and Sons.
Fain, A., Britt, D. P., & Molyneux, D. H. (1980). Myianoetus copromyzae sp. nov. (Acari, Astigmata, Anoetidae) phoretic on Copromyza atra (Meigen 1830) in Scotland. Journal of Natural History, 14, 401–403. https://doi.org/
10.1080/00222938000770341
Farish, D. J., & Axtell, R. C. (1971). Phoresy redefined and examined in Macrocheles muscaedomesticae (Acarina: Macrochelidae). Acarologia, 13, 16–29.
Greenberg, B., & Carpenter, P. D. (1960). Factors in phoretic association of a mite and fly. Science, 132, 738–739. https://doi.org/10.1126/science.132.3429.738
Hani, M., Thieven, U., & Perotti, M. A. (2018). Soil bulb mites as trace evidence for the location of buried money. Forensic Science International, 292, e25–e30. https://doi.org/10.1016/j.forsciint.2018.09.016
Heo, C. C., Teel, P. D., & OConnor, B. M. (2021). Acari community in association with delayed pig carrion decomposition. Experimental and Applied Acarology, 85, 223–246. https://doi.org/10.1007/s10493-021-00676-6
Hwang, C., & Turner, B. D. (2005). Spatial and temporal variability of necrophagous Diptera from urban to rural areas. Medical and Veterinary Entomology, 19, 379–391. https://doi.org/10.1111/j.1365-2915.2005.00583.x
Kamaruzaman, N. A. C., Mašán, P., Velásquez, Y., González-Medina, A., Lindström, A., Braig, H. R. et al. (2018). Macrocheles species (Acari: Macrochelidae) associated with human corpses in Europe. Experimental and Applied Acarology, 76, 453–471. https://doi.org/10.1007/s10493-018-0321-4
Margolis, L., Esch, G. W., Holmes, J. C., Kuris, A. M., & Schad, G. A. (1982). The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists). Journal of Parasitology, 68, 131–133. https://doi.org/10.2307/3281335
Miranda, R., & Bermúdez, S. (2008). Ácaros (Arachnida: Acari) asociados con moscas Calliphoridae (Diptera: Oestroidea) en tres localidades de Panamá. Revista Colombiana de Entomología, 34, 192–196. https://doi.org/10.25100/socolen.v34i2.9287
Mulieri, P. R., Mariluis, J. C., & Patitucci, L. D. (2014). Calliphoridae. In S. Roig-Juñent, L. E. Claps, & J. J. Morrone (Eds.), Biodiversidad de artrópodos argentinos, Vol. 4 (pp. 463–474). INSUE, Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina.
Negm, M. W., & Alatawi, F. J. (2011). Four new records of mites (Acari: Astigmata) phoretic on insects in Riyadh, Saudi Arabia. Journal of the Saudi Society of Agricultural Sciences, 10, 95–99. https://doi.org/10.1016/j.jssas.2011.04.001
OConnor, B. M. (1982). Evolutionary ecology of astigmatid mites. Annual Review of Entomology, 27, 385–409. https://doi.org/10.1146/annurev.en.27.010182.002125
OConnor, B. M. (2009). Astigmatid mites (Acari: Sarcoptiformes) of forensic interest. Experimental and Applied Acarology, 49, 125–133. https://doi.org/10.1007/s10493-009-9270-2
OConnor, B. M., Pimsler, M. L., Owings, C. G., & Tomberlin, J. K. (2015). Redescription of Myianoetus muscarum (Acari: Histiostomatidae) associated with human remains in Texas, USA, with designation of a neotype from Western Europe. Journal of Medical. Entomology, 52, 539–550. https://doi.org/10.1093/jme/tjv045
Olea, S. M., & Mariluis, J. C. (2013). The genus Calliphora (Diptera: Calliphoridae) in Argentina, with the first records of C. lopesi Mello 1962. Revista de la Sociedad Entomológica Argentina, 72, 99–104.
Perotti, M. A., & Braig, H. R. (2009). Phoretic mites associated with animal and human decomposition. Experimental and Applied Acarology, 49, 85–124. https://doi.org/10.1007/s10493-009-9280-0
Perotti, M. A., Braig, H. R., & Goff, M. L. (2010). Phoretic mites and carcasses: Acari transported by organisms associated with animal and human decomposition. In J. Amendt, M. Goff, C. Campobasso, & M. Grassberger (Eds.), Current concepts in forensic Entomology (pp. 69–91). Dordrecht: Springer. https://doi.org/10.1007/978-1-4020-9684-6_5
Perotti, M. A., & Braig, H. R. (2019). Acarology in Crimino-Legal Investigations. In J. Byrd, & J. Tomberlin (Eds.), Forensic Entomology, 3rd. Edition (pp. 461–473). Boca Raton: CRC Press. https://doi.org/10.4324/9781351163767-22
Pimsler, M. L., Owings, C. G., Sanford, M. R., OConnor, B. M., Teel, P. D., Mohr, R. M., & Tomberlin, J. K. (2016). Associa-
tion of Myianoetus muscarum (Acari: Histiostomatidae) with Synthesiomyia nudiseta (Wulp) (Diptera: Muscidae) on human remains. Journal of Medical Entomology, 53, 290–295. https://doi.org/10.1093/jme/tjv203
Rai, J., Amendt, J., Bernhardt, V., Pasquerault, T., Lindström, A., & Perotti, M. A. (2020). Mites (Acari) as a relevant tool in trace evidence and postmortem analyses of buried corpses. Journal of Forensic Sciences, 65, 2174–2183. https://doi.org/10.1111/1556-4029.14506
Rodrigueiro, T. S. C., & Prado, A. P. (2004). Macrocheles muscaedomesticae (Acari, Macrochelidae) and a species of Uroseius (Acari, Polyaspididae) phoretic on Musca domestica (Diptera, Muscidae): effects on dispersal and colonization of poultry manure. Iheringia. Série Zoologia, 94, 181–185. https://doi.org/10.1590/S0073-47212004000200011
Russell, D. J., Schulz, M. M., & OConnor, B. M. (2004). Mass occurrence of astigmatid mites on human remains. Abhandlungen und Berichte des Naturkundmuseums Görlitz, 76, 51–56.
Saloña-Bordas, M. I., & Perotti, M. A. (2015). Acarología forense. Ciencia Forense, 12, 91–112.
Szelecz, I., Lösch, S., Seppey, C. V. W., Lara, E., Singer, D., Sorge, F. et al. (2018). Comparative analysis of bones, mites, soil chemistry, nematodes and soil micro-eukaryotes from a suspected homicide to estimate the post-mortem interval. Scientific Reports, 8, 25. https://doi.org/10.1038/s41598-017-18179-z
Whitworth, T. (2014). A revision of the Neotropical species of Lucilia Robineau- Desvoidy (Diptera: Calliphoridae). Zootaxa, 3810, 1–76. https://doi.org/10.11646/zootaxa.3810.
1.1
Zamec, R., & Košel, V. (2014). A new species of mite (Acari: Histiostomatidae) phoretic on Gymnomus caesius (Diptera: Heleomyzidae) from Vlčie Diery cave. Biologia, 69, 916–919. https://doi.org/10.2478/s11756-014-0387-3