Omer José Jiménez-Ortega a, d, Keiner L. Tílvez b, Joselin Castro-Palacios a,
Andrés García c, *, Gabriel R. Navas a, Julio Abad Ferrer-Sotelo e, Dilia Naranjo-Calderón e, Juan Gabriel Díaz-Castellar e, Víctor Buelvas-Meléndez e
a Universidad de Cartagena, Campus San Pablo, Grupo de Investigación en Hidrobiología, Programa de Biología, Carrera 50#24-120, Zaragocilla, Cartagena de Indias, Colombia.
b Universidad de Cartagena, Campus San Pablo, Grupo de Investigación en Biología Descriptiva y Aplicada, Carrera 50#24-120, Zaragocilla, Cartagena de Indias, Colombia
c Universidad Nacional Autónoma de México, Instituto de Biología, Estación de Biología Chamela, Apartado postal 21, 48980 San Patricio, Jalisco, México
d Parque Temático Vivarium del Caribe-Fundación Archosauria zona norte km 15, Provincia de Cartagena, Bolívar, Colombia
e Santuario de Flora y Fauna Los Colorados, Parques Nacionales Naturales de Colombia, Carrera 8# 9-20 Plaza Olaya Herrera, San Juan Nepomuceno, Bolívar, Colombia
*Corresponding author: chanoc@ib.unam.mx (A. García)
Received: 28 October 2023; accepted: 18 March 2024
Abstract
This study aimed to determine anuran diversity and the use of microhabitats in 3 vegetation covers in the Santuario de Flora y Fauna Los Colorados. Five field trips of 6 days each were made, 2 days and 2 nights in each cover: forest, pasture, and crop. Sampling was carried out with the visual encounter inspection technique under a randomized design by random walks with manual capture. A total of 19 species were recorded, 14 in the forest, 13 in pasture, and 12 in crop. Pasture and crop were the vegetation covers with the greatest similarity of species. This work updates the list of anuran species recorded in the management plan of the Santuario de Flora y Fauna Los Colorados 2018-2023. The greatest number of anuran species was associated with leaf litter, “jagüeyes”, and soils. The transformation of the landscape as a result of agriculture and cattle ranching generated changes in the richness, abundance, composition, and use of microhabitats of the anurans present in the Santuario de Flora y Fauna Los Colorados.
Keywords: Landscape transformation; Vegetation coverage; Microhabitat; Tropical dry forest
Diversidad de anuros y uso de microhábitats en tres coberturas vegetales del Santuario de Flora y Fauna Los Colorados, Caribe colombiano
Resumen
Este estudio tuvo como objetivo determinar la diversidad de anuros y el uso de microhábitats en 3 coberturas vegetales en el Santuario de Flora y Fauna Los Colorados. Se hicieron 5 salidas de campo de 6 días cada una, 2 días y 2 noches en cada una: bosque, potrero y cultivo. Se realizaron muestreos con la técnica de inspección por encuentro visual, bajo el diseño aleatorizado por caminatas al azar con captura manual. Se registraron 19 especies, 14 de ellas en bosque, 13 en potrero y 12 en cultivo, siendo el potrero y el cultivo las coberturas con mayor similitud de especies. Este trabajo actualiza el listado de las especies de anuros registrados en el Plan de manejo del Santuario de Flora y Fauna Los Colorados 2018-2023. El mayor número de especies de anuros se encontró asociado a la hojarasca, el jagüey y los suelos. La transformación del paisaje producto de la agricultura y la ganadería genera cambios en la riqueza, abundancia, composición y uso de microhábitats de los anuros presentes en el Santuario de Flora y Fauna Los Colorados.
Palabras clave: Transformación del paisaje; Coberturas vegetales; Microhábitat; Bosque seco tropical
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/)
Introduction
Seasonally tropical dry forests (STDF here after) in Colombia are distributed mainly in the inter-Andean valleys and the Caribbean region (García et al., 2014), the latter being one of the regions with the best conserved areas of this ecosystem (Pizano et al., 2014; Rodríguez et al., 2012). However, Etter et al. (2008) point out that indiscriminate deforestation for various anthropogenic activities such as agriculture and livestock have generated large reductions in forest cover over time. This, combined with other activities such as mining and urban development (Cristal et al., 2020; Galván-Guevara et al., 2015; Jiménez et al., 2018), cause biological and ecological interactions to deteriorate, and the functionality of the ecosystem is compromised (Thomson et al., 2017), which is why Colombian STDFs have been classified as critically endangered (CR) (Etter et al., 2017). Consequently, it is a strategic ecosystem for conservation study due to its high risk of disappearing, strongly threatening the local fauna and the people who depend directly and indirectly on the ecosystem services it provides (Andrade, 2011).
One of most sensitive groups to forest transformation is amphibians, including anurans, which are highly dependent on humid places or sites with high water availability since most of their species have indirect development, permeable skin, and anamniote-type eggs (O’Malley, 2007). The spatial distribution and microhabitats use by anurans depend on the physiological requirements of each organism, and the available resources (Urbina-Cardona et al., 2006; Zug et al., 2009), as suggested by several studies showing many anuran species prefer forested areas (Cáceres-Andrade & Urbina-Cardona, 2009; García-R et al., 2005; Román-Palacios et al., 2016). Consequently, these species may be affected by anthropogenic disturbance, forest fragmentation, and loss (Cáceres-Andrade & Urbina-Cardona, 2009).
Forest transformation is among the main factors affecting anuran communities (Cáceres-Andrade & Urbina-Cardona, 2009; Marín et al., 2017; Romero, 2013; Vargas & Bolaños-L, 1999), causing around 38% of Colombian amphibians to be included under a category of endangered species and positioning Colombia as the country with the highest number of threatened species according to the second global review of amphibians (Re:wild, 2023). A study carried out by Duarte-Marín et al. (2018) in 3 habitats of the Selva de Florencia National Natural Park estimated that the covers with greater vegetation (forest and pine forest) presented greater richness and diversity of anurans than those covers with less vegetal complexity (pastures). This means land use and changes in vegetation cover are factors that influence amphibian species richness and diversity. Therefore, species that are not adapted to the new environmental conditions created by landscape transformation are eliminated from the assembly, negatively affecting the ecosystem processes in which they had intervention (Díaz et al., 2006).
Additionally, forest fragmentation has created barriers that prevent anuran dispersal, resulting in a decrease in their genetic diversity (De Sá, 2005). Furthermore, it has generated changes in the composition and abundance of anurans to an extent that depends on the levels of disturbance (Acuña-Vargas, 2016), with an increase in the penetration of light and winds along the perimeter of a forest remnants, coming from non-forest environments such as pastures, with the subsequent changes in microclimates (Echeverry et al., 2006; Galván-Guevara et al., 2015; Laurence & Gascon, 1997), phenomena known as the edge effect ( Rojas & Pérez-Peña, 2018). However, studies such as Blanco and Bonilla (2010) show that some transformed areas provide a greater number of microenvironments due to the modifications made by humans (e.g., creation of jagüeyes) and record greater richness and abundance of anurans species when compared to less transformed areas, which is known as intermediate disturbance theory (Conell, 1978). However, it must be considered that the species found in these areas have extensive plasticity to tolerate the environmental and structural gradients generated by anthropogenic disturbance, that is, they are resilient (Cáceres-Andrade & Urbina-Cardona, 2009).
Based on the above, the general objective of this research was to determine the diversity of anurans and their use of microhabitats in 3 vegetation covers within the Los Colorados Flora and Fauna Sanctuary (SFF Los Colorados), an important protected area in the Caribbean region of Colombia, which contributes to the understanding of how amphibians respond to changes in land use for agriculture and livestock, in order to provide information that can be useful for environmental entities to determine management and conservation policies for these organisms in landscape fragments.
The specific objectives are: 1) to determine the richness, abundance, diversity, and composition of anurans in 3 vegetation covers that are representative of the Los Colorados SFF; 2) to describe the use of the microhabitat by the species in each vegetation cover; 3) to analyze and compare the relationship between precipitation and environmental temperature with the richness, abundance, and diversity of species in each vegetation cover and; 4) to analyze and compare the alpha and beta diversity of anuran species in each vegetation cover.
We expect to record differences between the 3 types of vegetation cover, hypothesizing that due to the greater heterogeneity of an ecosystem in better conservation condition such as the tropical forest, it will register a greater richness and diversity of species and its species composition will differ with respect to the other covers. While the use of the microhabitat by the species will differ in each cover and will depend on the variety of natural or anthropogenic substrates existing in each site.
Materials and methods
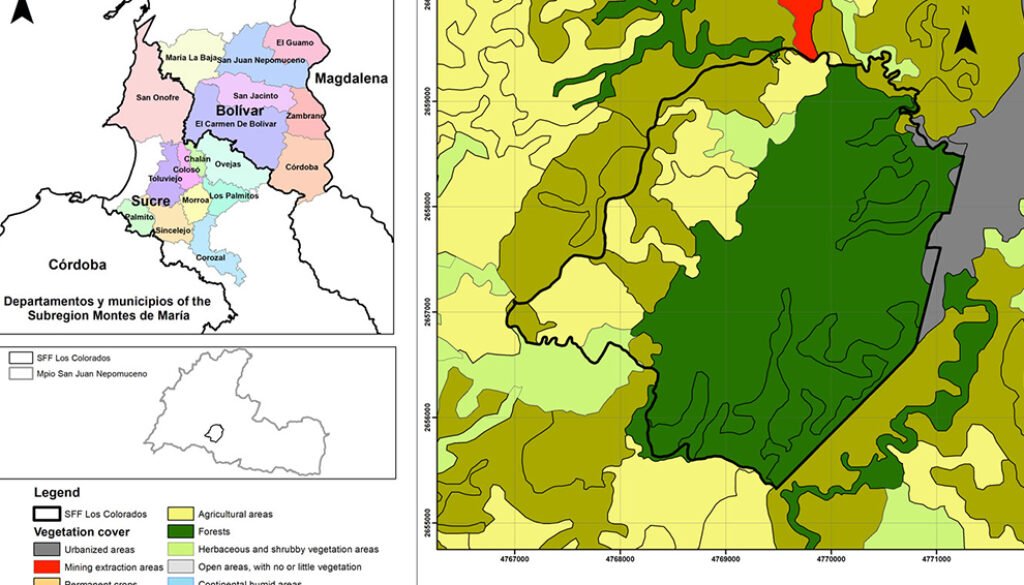
Montes de María is a subregion of the Colombian Caribbean. It is located between the departments of Sucre and Bolívar with an area of 6,297 km2, of which 3,719 km2 belong to the department of Bolívar (Aguilera-Díaz, 2013; Herazo et al., 2017). It integrates several municipalities, among which is San Juan Nepomuceno, where the SFF Los Colorados is located at 9°56’06.7” N, 75°06’48.7” W (Fig. 1) with an area of 1,041.96 ha, an average high temperature of 28 °C and an elevation of 23 m asl (Jiménez et al., 2018). Due to the seasonality of rainfall in the region, 3 seasons can be identified, each lasting 4 months and including the dry season (December to April), the transition season (little rain, May to August), and the rainy season (abundant rain, September to November). The average precipitation is around 1,643mm with a monthly average of 137mm (Rangel & Carvajal-Cogollo, 2012).
SFF Los Colorados is composed of a small mountain system formed by sedimentary rocks, in which the largest and most important STDF relic of Montes de María is located (Jiménez et al., 2018). This ecosystem has humid forest components, which is why it is considered a place of high species diversity (IAVH, 1998). Its hydrographic system is made up of 2 streams: Cacaos and Salvador, located on the south and north sides, respectively; it also has a large number of ravines that flow into these streams (Jiménez et al., 2018). There are 6 land uses within the SFF Los Colorados (Fig. 1), which are in descending order by their percentage of coverage, forest (66.36%), agricultural areas (17.29%), pastures (12.01%), herbaceous and shrubby vegetation areas (3.51%), urbanized areas (0.80%), and mining extraction areas (0.02%). The exact age of the crop areas is unknown; this area has historically been agricultural, even before 1977 when the SFF Los Colorados was declared as a protected natural area. However, for about 10 years these areas have been in the succession stage towards shrublands because they were purchased and practically little cultivated. There are only crops at the sampling point where yam (Dioscorea) or tuber is grown. The only management that is done with these crops is slash-and-burn. With respect to livestock, none of the pasture areas in the sampling sites have more than 40 heads of livestock. No fertilizers or other types of agrochemicals or pesticides are used.
SFF Los Colorados faces 2 main problems in the conservation of their natural environments. The first is an occupancy rate close to 30% of its surface (3 neighborhoods and 11 properties). The second is the inadequate environmental planning outside the protected area that has generated a transformation of the landscape because of cattle ranching, agriculture, forest plantations, mining activities, the presence of a national highway as a limit, and the proximity to a municipal seat of 25,000 inhabitants (Jiménez et al., 2018).
A two-day prospecting visit was carried out at the SFF Los Colorados in November 2021 to inspect the site and locate the sampling points. Subsequently, 5 field trips of 6 days each were carried out (2 days and 2 nights in each cover: forest, pasture, and crop) during the months of January, February, March, April, and June 2022. In this way, sampling was carried out during the dry and transition season, that is, under conditions of no rain (February to April) or very little rain (June). In these months 2 researchers and 2 officials from the SFF Los Colorados carried out daytime (8:00 -10:00 am) and nighttime (6:00-8:00 pm) outings with a constant speed route, for a sampling effort of 160 man-hours in each coverage for a total of 480 man-hours.
The visual encounter inspection technique was used to locate and record anuran species and their abundance, under the randomized design of random walks (Crump & Scott, 2001) and manual capture of individuals (Aguirre-León, 2011; Manzanilla & Péfaur, 2000). The identification of anuran species in each cover (forest, pasture, and crop) was based on regional taxonomic keys (Ballesteros-Correa et al., 2019; Cuentas et al., 2002; Dunn, 1994), supported by field guides with photographs (Meza-Tílvez et al., 2018; Salvador & Gómez-Sánchez, 2018), and databases (Acosta-Galvis, 2021).
The 3 selected coverages were described following the CORINE Land Cover methodology adapted for Colombia (IDEAM et al., 2008) as follows: forest is an area made up mainly of tree elements of native or exotic species, trees being woody plants with a single main trunk or in some cases with several stems, which also have a defined and semi continuous canopy. In the study area, trees reach a height greater than 5m, and watercourses with a width of less than 50m were found. Pasture includes lowlands covered with grasses and some scattered trees with a height greater than 5 m, which are located on hills and flat pastures in warm climates. Crops are areas dedicated primarily to the production of food, fiber, and other raw materials with permanent, transitional, or annual crops of avocado, chili and cassava. Temporary yam crops are mainly found in the study area.
To describe microhabitat used by anurans, the number of individuals of each species observed in one of the substrate types (leaf litter, branch, trunk, sites with the presence of water, rock, soil, herbaceous or shrubby vegetation) were recorded (Cáceres-Andrade & Urbina-Cardona, 2009).
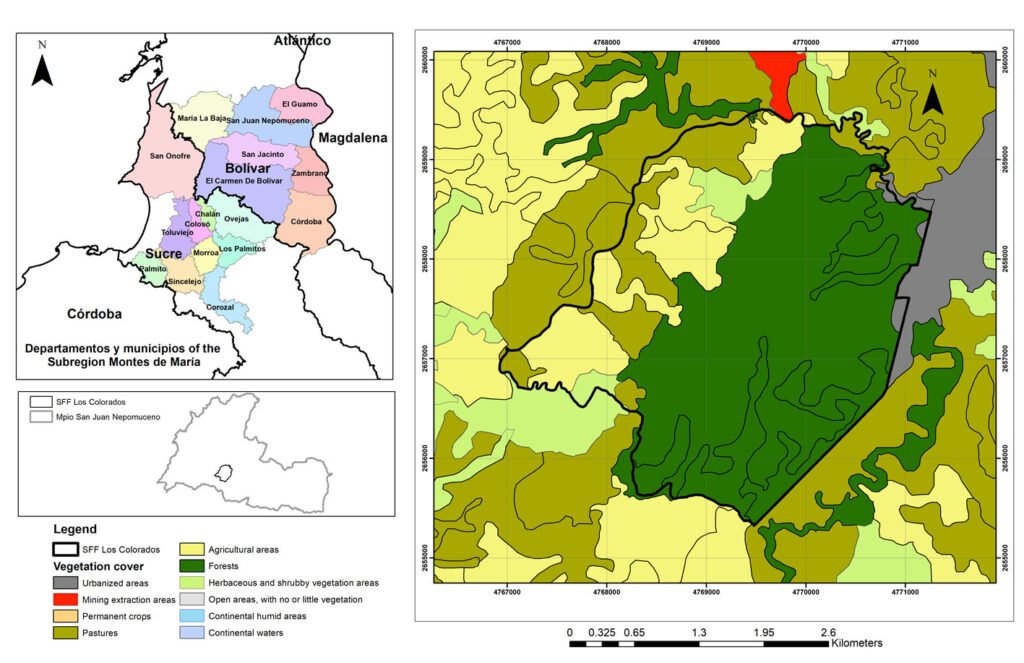
Figure 1. Location of the Los Colorados Flora and Fauna Sanctuary; source National Natural Parks of Colombia, with permission granted by SFF Los Colorados.
All observed species were photographed and at least 1 individual per species was collected, anesthetized with 2% xylocaine gel on the head and belly, and sacrificed (McDiarmid, 1994). To avoid tissue necrosis, they were prepared and fixed with 10% formalin (McDiarmid, 1994; Simmons & Muñoz-Saba, 2005), then placed in a suitable position in a container that had a white absorbent paper impregnated with 10% formalin. Distinctive characteristics were then observed. Finally, they were preserved in 70% ethanol (Cortez-F et al., 2006). The collected material was deposited in the Armando Dugand Gnecco collection of the Universidad del Atlántico, with the following catalog numbers: UARC-Am-00508, UARC-Am-00509, UARC-Am-00510, UARC-Am-00511, UARC- Am-00512, UARC-Am-00513, and UARC-Am-00514. The collecting permit was granted by the regional environmental authority called the Regional Autonomous Corporation of the Canal del Dique (Cardique), and the permit number is the resolution number 0751 of June 27, 2014. In addition, through the research endorsement approved by National Parks of Colombia, No. 20212000004933, October 25, 2021.
Information on the number of species and their abundance in each cover and climatic season was stored in an Excel. To confirm sampling was carried out on dates with the typical characteristics of the climatic seasons (rainy and dry), we graphed and compared statistically (ANOVA) the average precipitation and temperature for the months in which the sampling was carried out based on data obtained from the Institute of Hydrology, Meteorology and Environmental Studies (IDEAM) of the Guamo-Bolívar Station (Retrieved on July 19, 2022, from: http://www.ideam.gov.co/web/atencion-y-participacion-ciudadana/pqrs).
To detect significant differences in alpha diversity (richness, abundance, Simpson, Shannon), the Kruskall-Wallis or ANOVA tests were applied, depending on the normality of the data using the Shapiro-Wilk test and homogeneity of variances using Levene’s test (p < 0.05).
Alpha diversity was determined as the species richness for each coverage (Moreno, 2001), and was evaluated using Chao 1, 2, and Jack 1 estimators in EstimateS v. 9.1 (Villareal et al., 2004). In addition, bootstrap was used, which is useful to determine richness with a high number of rare species (Colwell & Coddington, 1994; Magurran, 2004). On the other hand, the diversity of anurans was estimated for each cover using the Shannon-Wiener index in the program PAST v. 4.03 (Hammer et al., 2001), and dominance using the Simpson index, where values close to 0 were considered as low levels of dominance and those close to 1 as high levels of dominance (Clarke et al., 2014).
To evaluate the turnover of anuran species between different covers (forest, pasture, and crops), the Jaccard index was used because it relates the number of shared species to the total number of exclusive species (Villareal et al., 2004). The range of values goes from 0 in the case of no shared species, to 1 when the covers have the same species composition (Moreno, 2001). From the estimator, a dendrogram was constructed in PAST v. 4.03.
To analyze the use of microhabitats, a graph was constructed where the percentage of use of each microhabitat by species and cover was established, to observe in each cover which microhabitats were most used by each species of anuran. Data were plotted in Excel.
Results
In total 1,269 individuals belonging to 19 species and 1 casual record (not included in this analysis) were recorded and grouped into 13 genera and 7 families (Table 1). Hylidae and Leptodactylidae were the families with the greatest species recorded, 8 and 6, respectively whereas only 1 species was recorded for Microhylidae and Phyllomedusidae.
Table 1
Taxonomic list and number of anuran individuals recorded in forest, crop, and pasture cover in the Los Colorados Flora and Fauna Sanctuary.
| Family | Species | Forest | Crops | Pasture |
| Bufonidae | Rhinella horribilis (Wiegmann, 1833) | 29 | 48 | 89 |
| Rhinella humboldti (Spix, 1824) | 2 | 97 | 101 | |
| Ceratophryidae | Ceratophrys calcarata (Boulenger, 1890)* | |||
| Dendrobatidae | Dendrobates truncatus (Cope, 1861, “1860”) | 173 | ||
| Hylidae | Boana platanera (Escalona et al., 2021) | 23 | 3 | 4 |
| Boana pugnax (Schmidt, 1857) | 3 | 90 | ||
| Dendropsophus ebraccatus (Cope, 1874) | 1 | |||
| Dendropsophus microcephalus (Cope, 1886) | 2 | 4 | 59 | |
| Scarthyla vigilans (Solano, 1971) | 3 | |||
| Scinax cf. rostratus (Peters, 1863) | 5 | 14 | ||
| Scinax cf. ruber (Laurenti, 1768) | 1 | 2 | ||
| Trachycephalus typhonius (Linnaeus, 1978) | 6 | 1 | 7 | |
| Leptodactylidae | Engystomops pustulosus (Cope, 1864) | 190 | 15 | 12 |
| Leptodactylus fuscus (Schneider, 1799) | 2 | 55 | ||
| Leptodactylus insularum (Barbour, 1906) | 12 | 1 | 81 | |
| Leptodactylus poecilochilus (Cope, 1862) | 44 | |||
| Leptodactylus savagei (Heyer, 2005) | 17 | |||
| Pleurodema brachyops (Cope, 1869, “1868”) | 38 | 10 | ||
| Microhylidae | Elachistocleis panamensis (Dunn et al., 1948) | 19 | ||
| Phyllomedusidae | Phyllomedusa venusta (Duellman & Trueb, 1967) | 1 | 5 | |
| * Species recorded casually outside the sampled coverage, which is not included in the analyses of our study. |
Table 2
Richness estimators and percentages of representativeness with respect to the number of anuran species recorded in the 3 coverages of the SFF Los Colorados.
| Richness estimator | Cover | ||
| Forest | Pasture | Crops | |
| Species recorded | 14 | 13 | 12 |
| Chao 1 | 15.00 (86.7%) | 13.00 (100.0%) | 12.33 (97.3%) |
| Chao 2 | 14.68 (88.6%) | 13.00 (100.0%) | 12.90 (93.0%) |
| Jack 1 | 16.70 (77.8%) | 13.90 (93.5%) | 14.70 (81.6%) |
| Bootstrap | 15.40 (84.3%) | 13.57 (95.8%) | 13.32 (90.1%) |
Alpha diversity was highest in forest (14 species), followed by pasture (13 species) and crop (12 species). The species accumulation curves in the 3 coverages based on the Chao 1, Chao 2, and bootstrap estimators allowed estimating a number of species similar to that recorded in the field and an efficiency in the sampling carried out with a representativeness greater than 80%. The Jack 1 estimator for forest indicates a representativeness of 77.8%, and for pasture and crops greater than 80% (Table 2, Fig. 2). In the singleton and doubleton curves (Fig. 2), a decreasing behavior is observed for the pasture and crop covers, indicating little probability of finding new anuran species in these covers. For forest, the doubleton curve shows an ascending behavior, indicating a probability of finding more species in this cover.
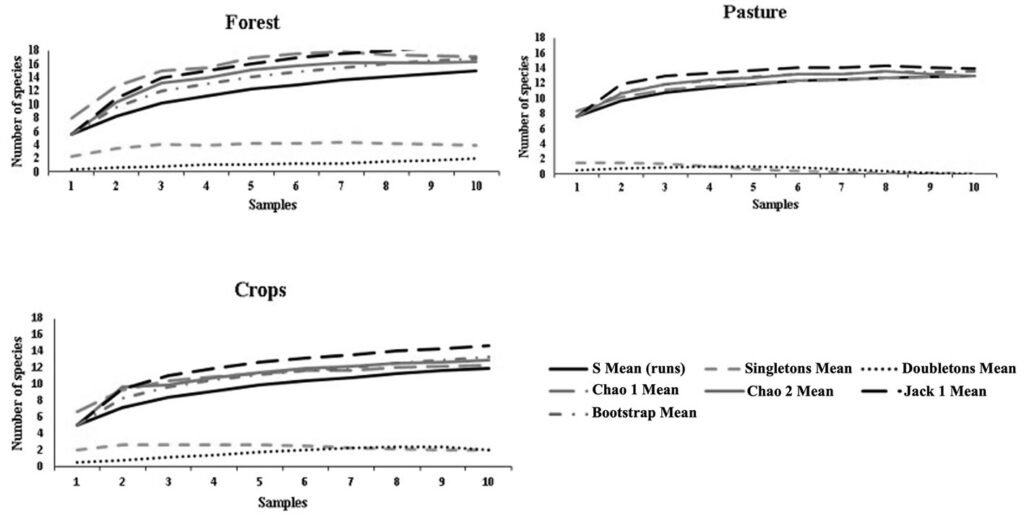
Figure 2. Accumulation curves of anuran species for the 3 coverages of the Los Colorados Flora and Fauna Sanctuary.

Figure 3. Box plots of richness (A), abundance (B), Shannon Index (C), and Simpson (D) for each of the 3 vegetation covers.

Figure 4. Histogram and box plot of daily ambient temperature across the sampling months; temperature (A, B), rainfall (C).
According to the determined Shannon-Wiener index, the diversity for forest cover was 1,631, crops 1,677, and pasture 2,107. On the other hand, Simpson’s index estimated a dominance of 0.726 for the forest, crops 0.746, and pasture 0.858. When comparing the metrics recorded in each vegetation cover (Fig. 3), the pastures registered on average the greatest richness, abundance, and diversity. The average richness was similar between the crops and the forest; however, the variation was greater in the crops. In contrast, the average and variation of abundance was greater in the forest than in the crops. The average species diversity was lowest in forests, intermediate in crops, and highest in pastures. There were statically differences of all metrics among vegetation cover; richness (one-way ANOVA, F = 5.456, df = 2, p > 0.05), abundance (H(χ2) = 4.63, p > 0.05), Shannon (one-way ANOVA, F = 16.71, df = 2, p > 0.05), and Simpson (H(χ2) = 15.97, p > 0.05).
When we graph the monthly fluctuations of ambient temperature and precipitation (Fig. 4), it is evident that during the days and months of sampling, precipitation was little or none (monthly average from 1.3 mm in January to 7.4 mm in April) whereas monthly average temperature tended to increase from 21.5 °C to 23.3 °C from January to March and from 24.0 to 24.5 °C from April to June. These daily temperature records showed significant monthly differences (ANOVA, F = 67.1, df5 = 5, df2 = 79.8, p < 0.001). There were no significant monthly fluctuations with respect to precipitation (ANOVA, F = 1.78, df5 = 5, df2 = 78.8, p > 0.05). The species richness tended to be higher in April and June in all 3 vegetation covers (Fig. 5) whereas abundance was higher in the forest in March and higher for pastures and crops during April and June. Species diversity (Shannon and Simpson) in the forest was higher in March whereas in both crops and pastures it was higher in June (Fig. 5).
Based on the Jaccard similarity index, crop and pastures presented a greater degree of similarity (Fig. 6), that is, a greater number of shared species. The forest presented the greatest dissimilarity in species composition with respect to the crop and the pasture, having a greater number of unique species (D. truncatus, D. ebraccatus, L. poecilochilus, and L. savagei), which are shown in Table 1.
It was observed that the microhabitat most used by anurans in the forest was leaf litter. The species most associated with this type of microhabitat were D. truncatus and L. poecilochilus (Fig. 7); these species were only recorded in this coverage (Table 1). In the pasture, the highest record of species was found in bare soils and jagüeyes, with L. fuscus, L. insularum, and B. pugnax being the most associated with the latter, while R. horribilis and R. humboldti were observed mainly in bare soil (Fig. 8). In addition, these species presented the highest number of records of individuals in this coverage (Table 1). Finally, in the crop coverage, the microhabitat with the highest number of anuran records was bare soil (Fig. 9), this microhabitat was used most frequently by R. humboldti, R. horribilis, and P. brachyops which were the species with the highest number of individuals recorded; this microhabitat was also used by E. panamensis, which was the only species present in this cover (Table 1).
Discussion
In this study, 19 species of anurans and 1 casual record were identified, for a total of 20 species, this being a slightly smaller number than the 21 species recorded in the SFF Los Colorados 2018-2023 Management Plan (Jiménez et al., 2018). Craugastor raniformis (Boulenger, 1896), Pseudopaludicola pusilla (Ruthven, 1916), and Lectodactylus fragilis (Brocchi, 1877) were not observed in our study, possibly due to lack of sampling in some areas of the SFF Los Colorados. Their occurrence cannot be ruled out, since they were recorded by Acosta-Galvis (2012) in the Montes de María. This study reports C. raniformis in the forest, in ravines (on rocks), on leaf litter, and in shrubby vegetation; P. pusilla in crop areas and on the edge of plain forests, on sandy substrates and in cracks after rains; L. fragilis in flat areas, around seasonal ponds, and near swamps.
Scarthyla, D. ebraccatus, and L. savagei are added to the anuran fauna of the SFF Los Colorados, which shows that it is necessary to continue carrying out studies in the subregions of STDF, including the protected areas of the plains of the Caribbean region, valleys of the Magdalena and Cauca Rivers, Catatumbo, and enclaves of the Patía Valley. Amphibian diversity is poorly known due to the lack of biological studies (Urbina-Cardona et al., 2014).
Sampling carried out in the first 3 months of the year (January, February, and March) regularly corresponds to the dry season (Rangel & Carvajal-Cogollo, 2012). However, rains occurring in these months is a consequence of the effects of the La Niña phenomenon in Colombia for 2022 (Guzmán-Ferraro & García, 2022).

Figure 4. Histogram and box plot of daily ambient temperature across the sampling months; temperature (A, B), rainfall (C).
Increases were observed in the specific richness and in the recorded number of individuals as rainfall increased (especially in April and June), so it was considered that the rainfall regime prior to sampling played an important role in the observation of anurans. These increases in richness and mainly in the number of individuals are attributed to higher activity and the reproductive strategies of some species, which take advantage of the rains to reproduce and lay eggs in temporary ponds. The rains caused greater activity and detectability of some species that were observed vocalizing in small ponds that had formed and cow dams.

Figure 5. Monthly trend of average richness (A), abundance (B), and diversity (C, D) for each vegetation cover type.
Only some amplexuses were recorded but we did not record nesting or reproduction events. Some of the species have explosive activity, which generates an increase in the number of individuals, as is the case of R. horribilis and other species (Vargas-Salinas et al., 2019); some other species vocalizing included Engytomops pustulosus in some ponds and Dendropsophus microcephalus in the emerging vegetation around the cow dams. However, it is worth mentioning that the frequency and intensity of the La Niña phenomenon due to climate change could alter the reproductive times of anurans, causing many species to have early reproduction, which would bring about temporal overlaps of the species that would generate changes in the structure of the assembly (Lawyer & Morin, 1993).
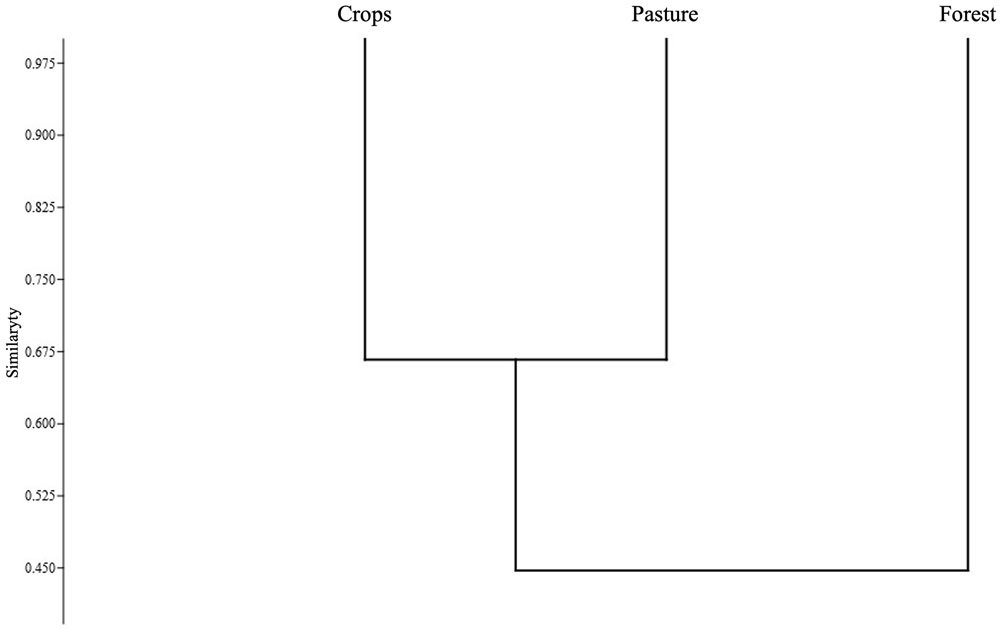
Figure 6. Jaccard similarity dendrogram for the anuran samples from the SFF Los Colorados.
As we expect, there were differences of species richness among vegetation covers. The forest recorded greater richness of anurans than the productive systems (pasture and crops). This is mainly attributed to the greater availability of humid microhabitats and the vegetaion complexity, since there are species that require dense vegetation cover and abundant leaf litter. For example, the oviposition of D. truncatus occurs in humid leaf litter (Cárdenas-Ortega et al., 2019), so different studies record it abundantly in forested areas (Burbano-Yandi et al., 2016; De la Ossa et al., 2016, 2011; Posso-Peláez et al., 2017). On the other hand, pasture and crop are covers with less complexity in the vegetal structure, generating changes in the composition of the anuran assemblages (Cortés-Gómez et al., 2013), such as the reduction in richness, which is closely linked to the reproductive modes of each species (Almeida-Gomes & Rocha, 2015). These same changes in richness in covers with different degrees of disturbance have been recorded in different studies carried out in the Middle Magdalena Valley, in Meta, and in Florencia (Burbano-Yandi et al., 2016; Cáceres-Andrade & Urbina-Cardona, 2009; Duarte-Marín et al., 2018). However, total and monthly average species richness and diversity tended to be higher in the pastures and the lowest in the forests, which registered the greatest monthly variation, recorded the higher species richness and diversity in April and June. The greater diversity of species recorded in pastures may be associated with the lower complexity of the vegetation structure of this habitat, which allows anurans to be easier to detect, while the greater structural complexity of forests and crops decreases detectability of the anurans. Additionally, the presence of jagüeyes in pastures, as sites with availability of water and constant humidity necessary for the survival of the anurans, contributed to the greatest number of records of individuals in this vegetation coverage. Leptodactylus fuscus, L. insularum, and B. pugnax had greater abundance in cow dams of the pasture, since they make postures close to bodies of water (Carvajal-Cogollo et al., 2019; Ortega-Chinquilla et al., 2019).

Figure 7. Use of microhabitats by species in the forest cover of the SFF Los Colorados.
Jagüeyes are considered important for many species in disturbed areas because they permanently provide water resources, which can be used to increase water uptake and reduce evaporation rates (Urbina-Cardona et al., 2014), in addition to be used by species with reproductive modes associated with this resource (Cardozo & Caraballo, 2017). On the other hand, bare soils were mostly used by R. horribilis and R. humboldti, which are species that are commonly found in disturbed areas (Acosta-Galvis, 2012), these have physical characteristics (tuberculated skin) and physiological characteristics that allow them to adapt to exploit this microhabitat (Cáceres-Andrade & Urbina-Cardona, 2009), for this reason, they were found with greater abundance in pastures and crops.
Dendrobates truncatus, D. ebraccatus, L. poecilochilus, and L. savagei are species that were recorded only in the forest, similar to what has been reported by other anuran assemblage studies, where they are not only recorded in forested areas, but also in wetlands (Acosta-Galvis et al., 2006; Angarita et al., 2015; Burbano-Yandi et al., 2016). On the other hand, S. vigilans was only recorded in pastures; in this study the species was observed mainly around bodies of water, specifically on emergent vegetation, in sympatry with D. microcephalus (Muñoz-Guerrero et al., 2007; Fonseca- Pérez et al., 2017). Finally, E. panamensis was only present in crops, although in the study carried out by Blanco-Torres et al. (2015), it was also recorded in pastures. This is a species identified as a leaf miner (Cuentas et al., 2002), which has possibly been the reason why it was observed near the cracks produced by cassava plantations. It is due to all the above that the species similarity analysis showed that the forest differs with respect to the other 2 covers, which are noticeably more similar to each other. Just as we expected, the forest differs in species composition.

Figure 8. Use of microhabitats by species in the pasture cover of the SFF Los Colorados.
The species diversity index values reported in our study are similar to those obtained in a nearby area located in Meta, Colombia (Cáceres-Andrade and Urbina-Cardona, 2009) where they reported values of 1.4 for humid forest, 1.43 in pastures and 1.9 in sugarcane crops. On the other hand, Román-Palacios et al. (2016) estimated a low Shannon-Wiener index in the Magdalena Medio for forest and quarry (0.92 and 1.74, respectively), while for the lake, they estimated an intermediate diversity (2.03). The forest value was very far from that estimated in this work, which may indicate that the SFF Los Colorados forest has an important conservation status that benefits the anurafauna.
On the other hand, Simpson’s index estimated high dominance for the 3 plant covers. This dominance may be associated with the microhabitats they offer; for example, the forest offered important microhabitats (numerous ponds and abundant leaf litter) for the development of E. pustulosus and D. truncatus, which made these species dominant in this cover. On the other hand, the crop was dominated mainly by P. brachyops, R. humbolbti, and R. horribilis; these species have terrestrial habits, tolerant to landscape transformations and abundant open environments (Acosta-Galvis, 2012; Rodríguez-Molina, 2004). Finally, the pasture was dominated by species of the genus Rhinella and Leptodactylus, where the latter has reproductive modes associated with foam nests, allowing them to conquer and be abundant in anthropized environments (Alcaide et al., 2012).
Jaccard’s similarity analysis for the anurans of the SFF Los Colorados indicates a grouping between pasture and crop cover due to the percentage of shared species (66.6%). This result may be associated with the fact that both covers are intervened areas, with a vegetation structure different from that of the forest and host generalist species (e.g., P. brachyops, B. pugnax, L. fuscus) that can share in greater quantities, while the forest, due to the resources it offers, may have species that do not tolerate landscape transformations (e.g., D. truncatus), being restricted only to forested areas (Cáceres-Andrade & Urbina-Cardona, 2009).
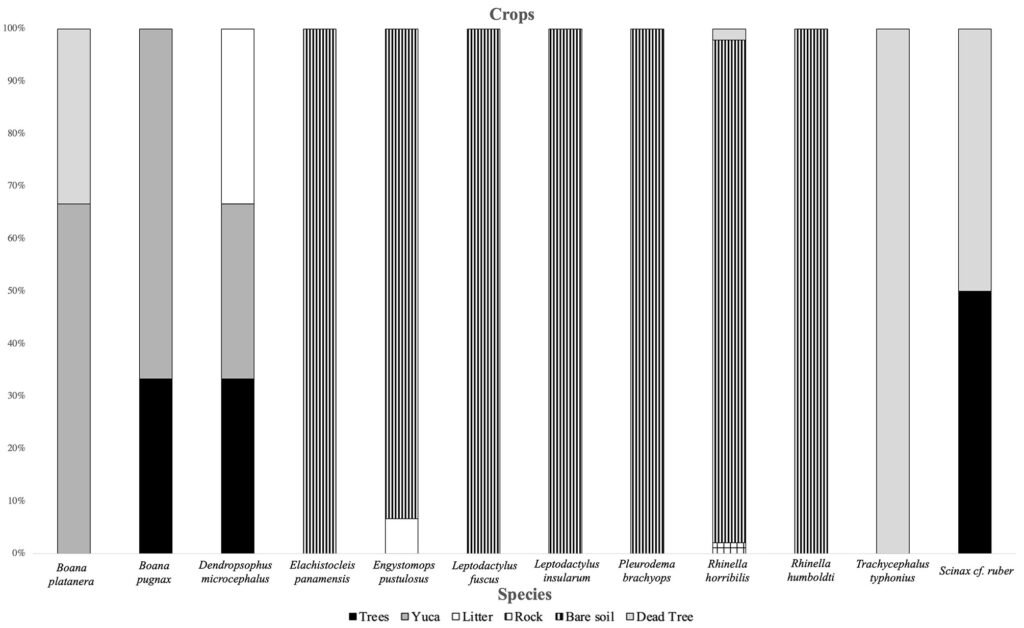
Figure 9. Use of microhabitats by species in the crop cover of the SFF Los Colorados.
The results of this research indicate that the transformation of the landscape because of the agricultural economy of the Montes de María, based mainly on cultivation and the raising of animals (Aguilera-Díaz, 2013), generated changes in the wealth, abundance, composition and use of microhabitats in anuran assemblages. Therefore, this knowledge is important to create concrete tools for the management and conservation of these organisms in the protected area and its surroundings, such as maintaining native vegetation and layers of leaf litter in productive systems, conserving lentic and lotic water sources, and reducing the use of agrochemicals, among others (Urbina-Cardona et al., 2015).
This research constitutes the baseline to evaluate the long-term response of anurans to ecological restoration processes and initiatives led by the SFF Los Colorados team in transformed areas of the protected area. This research constitutes the baseline to evaluate the long-term response of anurans to ecological restoration processes and initiatives led by the SFF Los Colorados team in transformed areas of the protected area. Results that could be useful in future studies where reference ecosystems (conserved areas) and disturbed areas in the process of restoration are used, to determine if these protected areas are achieving the expected objectives and if they are contributing to the conservation of anurans (Urbina-Cardona et al., 2015). Additionally, this study updates the list of anuran species in the protected area, pointing out those to a specific coverage and those shared among covers (forest, pasture, and crops), which can be useful to define those that may be vulnerable to fragmentation of the habitat or to be included as conservation target values (VOC) of the SFF Los Colorados in the construction of future Management Plans.
Acknowledgments
We thank the Hydrobiology research group of the University of Cartagena for providing their equipment for the development of this research. Likewise, to the University of Cartagena, for the financial support through resolution number 01878 of 2022. To IDEAM for providing information on the environmental variables for 2022. To Gabriel R. Navas-S, Dr. Andrés García, and Vivarium del Caribe for financial support and suggestions for carrying out this study. Likewise, to the technical and administrative team of the Los Colorados Flora and Fauna Sanctuary for their support in sampling, logistics, loan of facilities, and management of the research guarantee. To Joselin Castro-Palacios for his support in the implementation of the methodology of this work and to Adolfo A. Mulet-Paso for his suggestions in the identification of the species. To David Gernandt, for his revision to the text which improved substantially.
References
Acosta-Galvis, A. R. (2012). Anfibios de los enclaves secos del área de influencia de los montes de María y la ciénaga de La Caimanera, departamento de Sucre, Colombia. Biota Colombiana, 13, 211–255.
Acosta-Galvis, A. R. (2021). Lista de anfibios de Colombia. BATRACHIA. Retrieved on June 17, 2021 from: http://www.batrachia.com
Acosta-Galvis, A. R., Huertas-Salgado, C., & Rada, M. (2006). Aproximación al conocimiento de los anfibios en una localidad del Magdalena medio (Departamento de Caldas, Colombia). Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 30, 291–303. https://doi.org/10.18257/raccefyn.30(115).2006.2250
Acuña-Vargas, J. C. (2016). Anfibios y reptiles asociados a cinco coberturas de la tierra, municipio de Dibulla, La Guajira, Colombia. Acta Zoológica Mexicana, 32,133–146. https://doi.org/10.21829/azm.2016.322940
Aguilera-Díaz, M. M. (2013). Montes de María: una subregión de economía campesina y empresarial. Cartagena: CEER.
Aguirre-León, G. (2011). Métodos de estimación, captura y contención de anfibios y reptiles. Manual de Técnicas para el estudio de la Fauna. In S. López-González y C. López-González (Eds.), Manual de técnicas para el estudio de fauna (pp. 61–85). Querétaro: Universidad Autónoma de Querétaro, A.C.
Alcaide, A. P., Ponssa, M. L., Alcaide, F. P., & Alcaide, M. (2012). Histología de ovario en hembras vitelogénicas de Leptodactylus latinasus (Anura, Leptodactylidae). Acta Zoológica Lilloana, 56, 44–53.
Almeida-Gomes, M., & Rocha, C. F. (2015). Habitat loss reduces the diversity of frog reproductive modes in an Atlantic Forest fragmented landscape. Biotropica, 47, 113–118. https://doi.org/10.1111/btp.12168
Andrade, M. G. (2011). Estado del conocimiento de la biodiversidad en Colombia y sus amenazas. Consideraciones para fortalecer la interacción ciencia-política. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 35, 491–508.
Angarita, M. O., Montes-Correa, A. C., & Renjifo, J. M. (2015). Amphibians and reptiles of an agroforestry system in the Colombian Caribbean. Amphibian and Reptile Conservation, 8, 33–52.
Blanco, A., & Bonilla, M. (2010). Partición de microhábitats entre especies de Bufonidae y Leiuperidae (Amphibia: Anura) en áreas con bosque seco tropical de la región Caribe-Colombia. Acta Biológica Colombiana, 15,47–60.
Blanco-Torres, A., Duré, M., & Bonilla, M. A. (2015). Observaciones sobre la dieta de Elachistocleis pearsei y Elachistocleis panamensis en dos áreas intervenidas de tierras bajas del norte de Colombia. Revista Mexicana de Biodiversidad, 86,538–540. https://doi.org/10.1016/j.rmb.2015.04.031
Ballesteros-Correa, J., Vidal-Pastrana, C., & Ortega-León, A. M. (2019). Anfibios de Córdoba, Colombia. Córdoba: Fondo Editorial de Córdoba.
Burbano-Yandi, C. E., Gómez-Díaz, M. A., Gómez-Figueroa, A., Velásquez-Trujillo, D. A., & Bolívar-García, W. (2016). Ensamblaje de anfibios presentes en un bosque seco y en sistemas productivos, Valle Medio del Magdalena, Victoria y La Dorada, Caldas, Colombia. Revista de Ciencias, 20, 81–93.
Cáceres-Andrade, S. P., & Urbina-Cardona, J. N. (2009). Ensamblajes de Anuros de sistemas productivos y bosques en el Piedemonte Llanero, departamento del Meta, Colombia. Caldasia, 31, 175–195.
Cárdenas-Ortega, M. S., Gutiérrez-Cárdenas, P. D., Cifuentes-Ortegón, M., & Patiño-Gallego, A. M. (2019). Dendrobates truncatus (Cope, 1861) Rana venenosa de rayas amarillas. Catálogo de Anfibios y Reptiles de Colombia, 5, 32–41.
Cardozo, J., & Caraballo, P. (2017). Fauna anura (Amphibia: Anura) asociada a jagüeyes en dos localidades de la región Caribe colombiana. Revista Colombiana de Ciencia Animal-RECIA, 9, 39–47. https://doi.org/10.24188/recia.v9.nS.2017.519
Carvajal-Cogollo, J. E., Bernal-González, V., Paternina-Hernández, A., Muñoz-Ávila, J. N., & Vargas-Salinas, F. (2019). Uso de hábitat y reglas de ensamble: patrones y mecanismos. In M. H. Restrepo-Domínguez, E. Vera-López, Y. Bolívar-Suárez, S. G. Numpaque-Piracoca, O. Y. Acuña-Rodríguez, Z. Z. Ojeda-Pérez et al. (Eds.), Biología de los anfibios y reptiles en bosque tropical del norte de Colombia (pp. 297–338). Tunja: Editorial UPTC.
Clarke, K. R., Gorley, N. R., Somerfield, P. J., & Warwick, R. M. (2014). Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: PRIMER-E.
Colwell, R. K., & Coddington, J. A. (1994). Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 345,101–118. https://doi.org/10.1098/rstb.1994.0091
Conell, J. H. (1978). Diversity in tropical rain forests and coral reefs. Science, 199,1302–1310. http://dx.doi.org/10.1126/science.199.4335.1302
Cortés-Gómez, A. M., Castro-Herrera, F., & Urbina-Cardona, J. N. (2013). Small changes in vegetation structure create great changes in amphibian ensembles in the Colombian Pacific rainforest. Tropical Conservation Science, 6, 749–769. https://doi.org/10.1177/194008291300600604
Cortez, F. C., Suárez-Mayorga, A. M., & López-López, F. J. (2006). Preparación y preservación de material científico. In A. Angulo, J. V. Rueda-Almonacid, J. V. Rodríguez-Mahecha y E. La Marca (Eds.), Técnicas de inventario y monitoreo para los anfibios de la región tropical andina (pp. 173–218). Bogotá: Panamericana Formas e Impreso S.A.
Cristal, A., Sánchez, E., Romero, J., Leyva, J., Andrade, W., & Buelvas, C. (2020). ¿Qué hemos logrado con el proyecto de Conectividades Socio-Ecosistémicas? La evolución de la metodología de las 4Ps: avances y retos. In A. Cristal, M. Peña y J. Ferrer-Sotelo (Eds.), El proyecto de conectividades Socio-Ecosistémicas en los Montes de María, 2013–2020 (pp. 63–87). Bogotá-Colombia: Fundación Herencia Ambiental Caribe.
Crump, M., & Scott, N. (2001). Relevamiento por encuentros visuales. In W. R. Heyer, M. A. Donnelly, R. W. Diarmid, L. C. Hayek, & M. S. Foster (Eds.), Medición y monitoreo de la diversidad biológica: métodos estandarizados para anfibios (pp. 80–86). Chubut, Argentina: Editorial Universitaria de la Patagonia.
Cuentas, D., Borja, R., Lynch, J. D., & Renjifo, J. M. (2002). Anuros del departamento del Atlántico y norte de Bolívar. Barranquilla: Editorial Cencys.
De Sá, R. O. (2005). Crisis global de biodiversidad: importancia de la diversidad genética y la extinción de anfibios. Agrociencia, 9, 513.
De la Ossa, J., Contreras-Gutiérrez, J. C., & Campillo-Castro, J. (2011). Censo de Dendrobates truncatus (Anura, Dendrobatidae) en la reserva forestal protectora Serranía de Coraza, Montes de María, Sucre, Colombia. Revista Colombiana de Ciencia Animal-RECIA, 3, 339–343. https://doi.org/10.24188/recia.v3.n2.2011.407
Díaz, S., Fargione, J., Chapin, F. S., & Tilman, D. (2006). Biodiversity loss threatens human well-being. Plos Biology, 4, 1300–1305. https://doi.org/10.1371/journal.pbio.0040277
Duarte-Marín, S., González-Acosta, C., & Vargas-Salinas, F. (2018). Estructura y composición de ensamblajes de anfibios en tres tipos de hábitat en el Parque Nacional Natural Selva de Florencia, Cordillera Central de Colombia. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 42, 227–236. https://doi.org/10.18257/raccefyn.631
Dunn, E. R. (1944). Los géneros de anfibios y reptiles de Colombia. Caldasia, 2,497–529.
Echeverry, M. A., & Rodríguez, J. M. (2006). Análisis de un paisaje fragmentado como herramienta para la conservación de la biodiversidad en áreas de bosque seco y subhúmedo tropical en el municipio de Pereira, Risaralda Colombia. Scientia et Technica, 12, 405–410.
Etter, A., Andrade, A., Saavedra, K., Amaya, P., Cortés, J., Pacheco, C. et al. (2017). Lista Roja de Ecosistemas de Colombia, 2,1–6. https://doi.org/10.13140/RG.2.2.10861.08165
Etter, A., McAlpine, C., & Possingham, H. (2008). Historical patterns and drivers of landscape change in Colombia since 1500: a regionalized spatial approach. Annals of the Association of American Geographers, 98, 2–23. https://doi.org/10.1080/00045600701733911
Fonseca-Pérez, K. A., Molina, C., & Tárano, Z. (2017). Diet of Dendropsophus microcephalus and Scarthyla vigilans (Anura: Hylidae) at a locality in north-western Venezuela with notes on microhabitat occupation. Papéis Avulsos de Zoologia, 57,93–104. https://doi.org/10.11606/0031-1049.2017.57.07
Fox, B. J., Taylor, J. E., Fox, M. D., & Williams, C. (1997). Vegetation changes across edges of rainforest remnants. Biological Conservation, 82, 1–13. https://doi.org/10.1016/S0006-3207(97)00011-6
Galván-Guevara, S., Ballut-Dajud, G., & De La Ossa, J. (2015). Determinación de la fragmentación del bosque seco del arroyo Pechelín, Montes de María, Caribe, Colombia. Biota Colombiana, 16, 149–157.
García, R. J. C., Castro, H. F., & Cárdenas, H. H. (2005). Relación entre la distribución de anuros y variables del hábitat en el sector La Romelia del Parque Nacional Natural Munchique (Cauca, Colombia). Caldasia, 27, 299–310.
García, H., Corzo, G., Isaacs, P., & Etter, A. (2014). Distribución y estado actual de los bosques remanentes del bioma de bosque seco tropical en Colombia: insumos para su gestión. In C. Pizano, & H. García (Eds.), El bosque seco tropical en Colombia (pp. 228–251). Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt.
Guzmán-Ferraro, M., & García, G. (2022). El fenómeno de La Niña persistirá hasta casi la mitad del año. CVC. Retrieved on January 14th, 2022 from: http://www.cvc.gov.co/boletin-prensa-007-2022
Hammer, Ø., Harper, D. A., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9.
Herazo, F., Mercado, J., & Mendoza, H. (2017). Estructura y composición florística del bosque seco tropical en los Montes de María (Sucre-Colombia). Ciencia en Desarrollo, 8,71–8.
Instituto Alexander von Humboldt. (1998). El bosque seco tropical en Colombia B-sT. Grupo de Exploraciones y Monitoreo Ambiental. Bogotá, Colombia.
IDEAM (Instituto de Hidrología, Meteorología y Estudios Ambientales), IGAC (Instituto Geográfico Agustín Codazzi), & CORMAGDALENA (Corporación Autónoma Regional del río Grande de La Magdalena). (2008). Mapa de cobertura de la Tierra Cuenca Magdalena-Cauca: Metodología CORINE Land Cover adaptada para Colombia a escala 1:100.000. Bogotá: IDEAM/ IGAC/ CORMAGDALENA.
Jiménez, B., De la Rosa, N., & Naranjo, D. (2018). Plan de manejo del Santuario de Flora y Fauna Los Colorados. Parques Nacionales Naturales de Colombia. Santa Marta: Dirección Territorial Caribe.
Laurance, W. F., & Gascon, C. (1997). How to creatively fragment a landscape. Conservation Biology, 11, 577–579.
Lawler, S., & Morin, P. (1993). Temporal overlap, competition, and priority effects in larval anurans. Ecology, 71, 174–182. https://doi.org/10.2307/1939512
Magurran, A. (2004). Measuring biological diversity. Malden: Blackwell Publishing.
Manzanilla, J., & Péfaur, J. E. (2000). Consideraciones sobre métodos y técnicas de campo para el estudio de anfibios y reptiles. Revista de Ecología Latinoamericana, 7,17–30.
Marín, A. M., Ríos, L., Ríos, L., & Almario, J. (2017). Impacto de la actividad ganadera sobre el suelo en Colombia. Ingeniería y Región, 17, 1–12. https://doi.org/10.25054/issn.2216-1325
Meza-Tílvez, K., Mulet-Paso, A., & Zambrano-Cantillo, R. (2018). Fauna del Jardín Botánico de “Guillermo Piñeres” de Cartagena, Turbaco, Colombia: Anfibios y reptiles. Versión 1. Chicago, Illinois: Field Museum.
Moreno, C. E. (2001). Métodos para medir la biodiversidad. Volumen 1. Zaragoza: CYTED, ORCYT/ UNESCO & SEA.
Muñoz-Guerrero, J., Serrano, V. H., & Ramírez-Pinilla, M. P. (2007). Uso de microhábitat, dieta y tiempo de actividad en cuatro especies simpátricas de ranas hílidas neotropicales (Anura: Hylidae). Caldasia, 29, 413–425.
McDiarmid, R. W. (1994). Preparing amphibians as scientific specimens. In W. R. Heyer, M. A. Donnelly, R. W. McDiarmid, L. C. Hayek, & M. S. Foster (Eds.), Measuring and monitoring biological diversity. standard methods for amphibians (pp: 289–296). Washington D.C.: Smithsonian Institution Press.
O’Malley, B. (2007). Anatomía y fisiología clínica de animales exóticos. Zaragoza: Servet.
Ortega-Chinquilla, J., Méndez-Narváez, J., Carvajalino-Fernández, J., & Galindo-Uribe, D. (2019). Ecofisiología. In F. Vargas-Salinas, J.A. Muñoz-Avila & M.E. Morales-Puente (Eds.), Biología de los anfibios y reptiles en bosque tropical del norte de Colombia (pp. 297–338). Tunja: Editorial UPTC.
Pizano, C., Gonzáles, R., Gonzáles, M., Castro-Lima, R., Rodríguez, N., Idárraga, A. et al. (2014). Plantas de los bosques secos de Colombia. In C. Pizano, & H. García (Eds.), El bosque seco tropical en Colombia (pp. 228–251). Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt.
Posso-Peláez, C., Blanco-Torres, A., & Gutiérrez-Moreno, L. C. (2017). Uso de microhábitats, actividad diaria y dieta de Dendrobates truncatus (Cope, 1861) (Anura: Dendrobatidae) en bosque seco tropical del norte de Colombia. Acta Zoológica Mexicana, 33, 490–502.
Rangel, C. J. O., & Carvajal-Cogollo, J. E. (2012). Clima de la región Caribe Colombiana. In J. O. Rangel (Ed.), Colombia diversidad biótica XII: la región Caribe de Colombia (pp. 67–129). Bogotá: Instituto de Ciencias Naturales.
Re:wild, Synchronicity Earth, IUCN SSC Amphibian Specialist Group. (2023). State of the World’s Amphibians: The Second Global Amphibian Assessment. Texas: Re:wild. Electronic source at the IUCN website
Rodríguez-Molina, C. R. (2004). Reproducción de Pleurodema brachyops (Anura: Lectodactylidae) en los llanos del Estado Apure, Venezuela. Memoria de la Fundación La Salle Ciencias Naturales, 2002, 117–125.
Rodríguez, G. M., Banda, K., Reyes, S. P., & Estupiñán-González, A. C. (2012). Lista comentada de las plantas vasculares de bosques secos prioritarios para la conservación en los departamentos de Atlántico y Bolívar (Caribe colombiano). Biota Colombiana, 13, 7–39.
Rojas, R. R., & Pérez-Peña, P. E. (2018). Evidencia preliminar del efecto borde en anfibios de la Reserva Nacional Pucacuro, al norte de la Amazonía peruana. Revista del Instituto de Investigaciones de la Amazonía Peruana, 27, 55–67.
Romero, H. G. (2013). Deforestación en Colombia: retos y perspectivas. In F. Dane (Ed.), El desafío del desarrollo sustentable en América Latina (pp. 123–142). Río de Janeiro: SOPLA.
Román-Palacios, C., Fernández-Garzón, S., Hernández, M., Ishida-Castañeda, J., Gallo-Franco, W., & Bolívar-García, A. (2016). Uso de microhábitat por anuros en un fragmento de bosque seco intervenido del Magdalena Medio, Guarinocito, Caldas. Boletín Científico Centro de Museos Museo de Historia Natural, 20, 181–196. https://doi.org/10.17151/bccm.2016.20.2.14
Salvador, J., & Gómez, D. (2018). Reptiles y anfibios del departamento del Atlántico, Colombia. Versión 3. Bogotá, Colombia: Field Museum-Museo de Historia Natural ANDES.
Stuart, S. N., Chanson, J., Cox, N.A., & Young, B. E. (2006). Estado global de los anfibios. In A. Angulo, J. V. Rueda-Almonacid, J. V. Rodríguez-Mahecha, & E. La Marca (Eds.), Técnicas de inventario y monitoreo para los anfibios de la región tropical andina (pp. 19–41). Bogotá: Panamericana Formas e Impreso S.A.
Simmons, J. E., & Muñoz-Saba, Y. (Eds.). (2005). Cuidado, manejo y conservación de las colecciones biológicas. Bogotá: Universidad Nacional de Colombia.
Thompson, P. L., Rayfield, B., & González, A. (2017). Loss of habitat and connectivity erodes species diversity, ecosystem functioning, and stability in metacommunity networks. Ecography, 40, 98–108. https://doi.org/10.1111/ecog.02558
Urbina-Cardona, J. N., Olivares-Pérez, M., & Reynoso, V. H. (2006). Herpetofauna diversity and microenvironment correlates across a pasture-edge-interior ecotone in tropical rainforest fragments in the Los Tuxtlas Biosphere Reserve of Veracruz, Mexico. Biological Conservation, 132,61–75. https://doi.org/10.1016/j.biocon.2006.03.014
Urbina-Cardona, J. N., Arturo-Navas, C., Gonzales, I., Gómez-Martínez., M. J., Llano-Mejía, J., Medina-Rangel, G. F. et al. (2014). Determinantes de la distribución de los anfibios en el bosque seco tropical de Colombia: herramientas para su conservación. In H. Pizano, & H. García (Eds.), El bosque seco tropical en Colombia (pp. 169–195). Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt.
Urbina-Cardona, J. N., Bernal, E. A., Giraldo-Echeverry, N., & Echeverry-Alcendra, A. (2015). El monitoreo de herpetofauna en los procesos de restauración ecológica: indicadores y métodos. Monitoreo a procesos de restauración ecológica, aplicado a ecosistemas terrestres. Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humbold.
Vargas-Salinas, F., Angarita-Sierra, T., Ospinal, L. A., Rocha-Úsuga, A., & Rueda-Solano, L. (2019). Comunicación y ecología reproductiva. In F. Vargas-Salinas, J.A. Muñoz-Avila & M.E. Morales-Puente (Eds.), Biología de los anfibios y reptiles en bosque tropical del norte de Colombia (pp. 297–338). Tunja: Editorial UPTC.
Vargas, S. F., & Bolaños, L. M. E. (1999). Anfibios y reptiles presentes en hábitats perturbados de selva lluviosa tropical en el bajo Anchicayá, Pacífico colombiano. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 23,499–511.
Villareal, H. M., Álvarez, M., Córdoba-Córdoba, S., Escobar, F., Fagua, G., Gast, F. et al. (2004). Manual de métodos para el desarrollo de inventarios de biodiversidad. Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt.
Zug, G. R., Vitt, L., & Caldwell, J. P. (2001). Herpetology: an introductory biology of amphibians and reptiles. San Diego: Academic Press.