Meghan I. Zolá-Rodríguez a, Mariana Cuautle b, *, Marco Daniel Rodríguez-Flores c, Citlalli Castillo-Guevara b
a Akumal Monkey Sanctuary & Rescued Animals, Camino a Uxuxubi s/n Predio Santa Pilar Lote 16, 77776 Akumal, Quintana Roo, Mexico
b Universidad Autónoma de Tlaxcala, Centro de Investigación en Ciencias Biológicas, Km 10.5 Carretera Tlaxcala-San Martín Texmelucan, 90120 San Felipe Ixtacuixtla, Tlaxcala, Mexico
c Blue Marlin Conservation (Conservation Diver), Sunset Beach Gili Air, Gili Indah, Pemenang, North Lombok Regency, West Nusa Tenggara 83355, Indonesia
*Corresponding author: mcuautle2004@gmail.com (M. Cuautle)
Received: 26 June 2024; accepted: 27 August 2024
Abstract
This study examines the impact of disturbance on the food preferences and dominance of an ant community in a temperate ecosystem in Mexico. The study focused on 2 types of vegetation: native oak forest and induced grassland (disturbed vegetation). Observations were conducted to record the food elements carried by ants to their nests. These data were analyzed using x2 tests. Tuna and honey baits were placed near the nests to record the presence of ants in 5-minute periods. We used a binomial model to determine whether the probability of finding an ant foraging at the baits was affected by vegetation type, bait type, and/or ant species. Additional baits were used to determine the ant dominance indices. T-tests and ANOVAs were used to compare dominance indices between vegetation types, baits, and ant species. No significant differences were observed in food preferences between vegetations. However, some species showed a preference for honey (i.e., carbohydrates), which could be limited in ground-level environments. Ants showed a submissive behavior in both vegetation types. This research shows that ants could optimize their nutrient intake, enabling them to survive efficiently even when facing disturbances, instead of increasing dominance.
Keywords: Ant nest; Dominance index; Feeding habits; Compensation hypothesis; Carbohydrates; Proteins
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Impacto del disturbio en las preferencias alimentarias y dominancia de las hormigas (Hymenoptera: Formicidae) en un bosque templado de México
Resumen
Este estudio examina el impacto del disturbio en las preferencias alimentarias y dominancia de una comunidad de hormigas en un ecosistema templado en México, en bosque de encino nativo y pastizal inducido (vegetación perturbada). Se registraron los alimentos transportados por las hormigas a sus nidos. Estos datos fueron analizados utilizando pruebas de x2. Se colocaron cebos de atún y miel cerca de los nidos para registrar la presencia de hormigas. Utilizamos un modelo binomial para determinar si la probabilidad de encontrar una hormiga en los cebos se veía afectada por la vegetación, cebo o especie de hormiga. Los índices de dominancia se determinaron usando cebos. Se emplearon pruebas t y Anova para comparar los índices de dominancia entre tipos de vegetación, cebos y especies de hormigas. No hubo diferencias significativas en las preferencias alimentarias entre tipos de vegetación, pero algunas especies mostraron una preferencia por la miel (carbohidratos), que podría ser un recurso limitado a nivel del suelo. Las hormigas mostraron un comportamiento sumiso en ambos tipos de vegetación. Esta investigación muestra que las hormigas podrían optimizar su ingesta de nutrientes, permitiéndoles sobrevivir bajo condiciones de disturbio, en lugar de aumentar su dominancia.
Palabras clave:Nidos de hormigas; Índice de dominancia; Hábitos alimenticios; Hipótesis de compensación; Carbohidratos; Proteínas
Introduction
Land use change stands as one of the primary factors contributing to global change and its adverse effects on biodiversity (Ellis et al., 2010; Foley et al., 2005; Sala et al., 2000). Land use change has reduced biodiversity through the loss, modification, and fragmentation of habitats; degradation of soil and water; and overexploitation of native species (Foley et al., 2005), and its effects depend strongly on the type, severity, frequency and timing of disturbance (Foley et al., 2005; White & Jentsch, 2001). Ants are one of the most dominant insects both ecologically and numerically (Rico-Gray & Oliveira, 2007; Schultheiss et al., 2022; Toro et al., 2012) that participate in different ecological processes —e.g., nutrient recycling, soil formation, decomposition, seed dispersion (Toro et al., 2012). Ants are model organisms to study the effect of disturbance because they respond to environmental change (Agosti et al., 2000; Andersen, 2000). It is often observed that disturbances favor behaviorally dominant ants, including invasive species. Vonshak and Gordon (2015), observed that native ant richness was highest in natural habitats, and alien species richness was highest in urban habitats, along an urban-rural gradient in the San Francisco Bay area. Nevertheless, the specific effect will depend on factors like the type of disturbance, including fires, floodings, or even treefall gaps (Cerdá et al., 2013). Hoffman and Andersen (2003) studied the response of ant functional groups to disturbance finding that the dominant Dolichoderinae and “hot climate specialists” tend to be favoured by low levels of disturbance. The opportunist and generalized Myrmicinae have wide habitat tolerances but are sensitive to competitive interactions, while “cryptic species” and “specialist predators” are highly sensitive to disturbance.
Feeding habits and foraging strategies represent vital life history traits among ants, playing a significant role in their ecological dynamics. These traits are contingent upon the availability of resources within their respective habitats (Andersen, 2000; Davidson, 2005), and the ensuing competition for those resources (Arnan et al., 2012). The feeding habits of ants play a pivotal role in determining their ecological function within the ecosystem (Spotti et al., 2015). However, despite their significance, the food habits and preferences of ants remain relatively understudied and represent one of the least understood aspects of their biology (Houdria et al., 2015).
The effects of disturbance (e.g., land use change) on ant feeding habits (especially foraging strategies) have not been studied in depth (Castillo-Guevara et al., 2019; Hernández-Flores et al., 2016; Radnan et al., 2018), and the way ants respond to habitat complexity can provide clues as to how ants could respond to disturbance. When the external disturbing factors are of high intensity (natural-fires or anthropogenic-deforestation), the disturbances can initiate a directional regression manifested as gradual or rapid simplification of the horizontal and vertical structure of a community, leading to the replacement of complex communities by a simpler one (Łaska, 2001). The level of habitat complexity can influence ant foraging patterns. For instance, higher complexity may decrease interspecific interactions and ant recruitment or minimize the trade-off between resource discovery and dominance (Parr & Gibb, 2012).
Examining the effect of soil surface complexity on food exploitation in the context of change from grassland to shrubland in Australia, Radnan et al. (2018) discovered that substrate complexity (wood debris, leaf litter, or no substrate) influenced the discovery time, ant size, and monopolization index of tuna and honey baits within testing arenas. However, at a larger scale of vegetation type, the effect was not observed. Similarly, Castillo-Guevara et al. (2019) found that the dominance level was similar in natural oak vegetation in comparison to an agricultural area in a temperate community; however, ant foraging strategies differed between the 2 communities.
Competition has been regarded as the primary factor influencing the structure of ant communities (Cerdá et al., 2013). Through the use of aggressive strategies, ants exert influence on the spatial distribution, abundance, and behavior of other ants. These strategies encompass a range of tactics, including the deployment of repellent chemicals and the establishment of territories (Cerdá et al., 2013). Moreover, ants can establish interspecific dominance hierarchies primarily based on variations in food collection behavior and aggressiveness. While there are various proposals for categorizing such hierarchies, the classification proposed by Vepsäläinen and Pisarski (1982) and Savolainen et al. (1989), as described by Cerdá et al. (2013), is considered the most well-defined hierarchical system from an ecological perspective. This classification system is founded on the aggressive behavior of ants and its impact on other ant species, and includes 3 categories: the dominant, subdominant and subordinate category. Dominant species exhibit highly aggressive behavior, exhibit numerical predominance over other species, fiercely defend their territories, and establish mutual exclusivity within their ecological communities. Subdominant species, while not actively defending territories, display a remarkable propensity for aggressively safeguarding their food resources (Cerdá et al., 2013). Lastly, subordinate species, characterized by small colonies devoid of recruitment systems, employ strategies to avoid physical confrontations with other colonies and species. Nonetheless, they exhibit a strong commitment to defending their nests against potential threats (Cerdá et al., 2013). Different foraging strategies, such as the subordinate species’ ability to discover resources before dominant species (dominance-discovery trade-off, Fellers, 1987) or their capacity to forage across a broader temperature range compared to dominant species —dominance-thermal tolerance trade-off (Fellers, 1989), can contribute to the coexistence of ants within a community.
The present study focuses on the analysis of ant feeding habits and dominance hierarchy in response to land use change within a temperate ecosystem located in central Mexico. A habitat with the native oak forest vegetation (complex habitat), was compared to a nearby area where the land use had been altered to induced grassland (simplified habitat). The study hypothesized: i) it is expected that in the oak forest, food items transported to the nest will be more varied than in the induced grassland. This variation at the species level is also anticipated due to the change in land use. The conversion from a complex habitat (oak forest) to a simplified habitat (induced grassland) will likely provide fewer resources variety for the ants; ii) it is expected that as a result of the disturbance in the induced grassland, ants will be more generalized in low heterogeneous habitats due to the dominance of generalist ant species. Additionally, the arrival times at the baits will be shorter due to the absence of leaf litter; iii) a lower dominance index is expected in the induced grassland than in the oak forest, due to the presence of subdominant species and the modification of the dominance hierarchy system by the land use change.
Materials and methods
The study was conducted within “Flor del Bosque” State Park, located in the municipality of Amozoc de Mota, in the state of Puebla. The coordinates of this protected reserve are 19°00’00”-19°01’50” N, 98°20’35”-98°20’53” W. The State Park encompasses an area of 664.03 hectares, characterized by altitudes ranging from 2,225 to 2,400 m asl. The annual average temperature in this region fluctuates between 14 °C and 16 °C, with the majority of rainfall occurring during the summer months, contributing to an average annual precipitation of 750 to 950 mm. It is important to note that the park experiences a distinct dry season lasting approximately 6 months, from November to April, as reported by Costes et al. (2006). The native vegetation of the reserve primarily consists of oak forest [Quercus castanea (Née), Q. laurina (Bonpl.), Q. laeta (Liebm.) (Fagaceae)]. However, human disturbances have led to the presence of induced grassland areas and, to a lesser extent, eucalyptus plantations [Eucalyptus spp. L´Hér) (Myrtaceae)] within the park, as documented by Costes et al. (2006). Given that the disturbed vegetation within the reserve primarily consisted of induced grassland, our research aimed to establish a meaningful comparison between native and altered vegetation.
Ant communities were surveyed once a month during specific periods (April, August and October 2015, and January to March 2016). The survey methodology involved the establishment of 6 transect plots (400 m × 20 m), with 3 plots located in the oak forest and 3 in the induced grassland. The spatial distribution of these transects can be observed in Figure 1. To locate ant nests, the transects were traversed, and various substrates such as leaf litter, stones, trunks, and branches were lifted and examined. Additionally, a total of 5 tuna baits and 5 honey baits were placed along the transects at 10 m intervals to attract ants and to follow them to locate their nest. The nests found during the survey were georeferenced for accurate spatial documentation. A 5-minute observation period was designated for each nest, wherein all food items transported by the ants to their nests were recorded. These observations were conducted between 9:00 a.m. and 3:00 p.m. on the sampling days. Each recorded item was subsequently categorized into one of the following groups: 1) plant elements, encompassing any component of plants except for seeds, 2) seeds, and 3) arthropods. After each observation period, 2 bait samples, enclosed within Petri dishes, were positioned in close proximity to the nests (approximately 10 cm), maintaining a distance of approximately 5 centimeters from each other. Various studies exploring the feeding habits, preferences, and foraging strategies of ants have employed diverse bait types. Typically, these baits consist of carbohydrates such as honey and other sources representing proteins such as tuna (Houdria et al., 2015; Lynch et al., 1980; Spotti et al., 2015; Trigos-Peral et al., 2016). The bait materials employed in the study consisted of honey and tuna, which were consistently provided in a standardized amount of one tablespoon, equivalent to approximately 5 grams. To quantify the number of individual ants on each type of food resource, we placed baits and observed them for 5 min. Ants responded very quickly to the baits; therefore, the observation time was limited to 5 min. Within this duration, the number of individuals was recorded for each bait type. After the observation periods, 1 to 3 ants were collected from each nest using a vacuum cleaner or tweezers and then preserved in Eppendorf tubes containing 70% ethanol. In the laboratory, the ants were separated, mounted, and identified to the genus level using the key by Mackay and Mackay (1989). In certain instances, the species identification was feasible by comparing the collected specimens with those present in the Entomological Collection of the Universidad de las Américas Puebla (UDLAP).
To analyze the food preferences of the ant community, we considered 3 factors. First, we determined whether the number of foragers carrying plants, seeds, or arthropods to the nest differed between the whole ant communities of each vegetation type or among ant species. We used the appropriate contingency tables and chi-square tests for such comparisons. Second, we determined whether the presence of foragers at each bait during each minute of the 5-minute period was influenced by vegetation type, bait type (honey or tuna), ant species or interactions among these factors. We used a binomial model to evaluate this response variable, with “ant presence” coded as 1 and “absence” as 0. To select the most relevant explanatory variables, we employed a stepwise forward approach. We began with the simplest model and successively added each response variable. The significance of adding each variable was assessed using an x2 test, comparing the previous model with the new model. If a variable was found to be statistically significant, it was retained in the model. Otherwise, it was removed, and we proceeded with the next explanatory variable. This stepwise procedure allowed us to build the final model with the most significant factors explaining the presence of foragers at each bait. No overdispersion was detected in the selected model. Post-hoc analyses were conducted when significant differences were detected. The statistical analysis of data was performed using R software (R Core Team, 2022).
We evaluated the dominance hierarchy of the ants in each vegetation type (oak forest and induced grassland), ant species, and bait types using the same transects as for the detection of the nests (Fig. 1), during October 2015 and January, February and March (2016). At each transect, 9 sampling points were established on the ground, spaced 10 m apart. Each sampling point consisted of a pair of Petri dishes with baits: one with honey and one with tuna, placed less than 5 cm apart. This resulted in a total of 540 baits (5 replicates × 6 transects × 9 sampling points × 2 baits; 2 samplings in February). These types of baits have been widely used for ant dominance hierarchy studies (Dáttilo et al., 2014; Parr & Gibb, 2012; Trigos-Peral et al., 2016).
For the first 3 sets of bait, we recorded the arrival times of the ants during a one-hour observation period. Most of the ant species were identified in the field. For the ants that were not identified, a few individuals (2 to 3 specimens) were collected. In the case of the remaining 6 sets of bait, they were filled with water. Ants that fell onto the Petri dish after 2 h were collected. The presence of water did not deter the ants from visiting, but it allowed us to determine which ants had been attracted to the baits. The baits were placed and retrieved from different transects in the field between 9:00 a.m. and 5:00 p.m. The order of the transects was changed on different sampling days to mitigate the potential impact of the time of day. Ant specimens collected from the baits were preserved in 70% ethanol and transported to the laboratory for further identification.
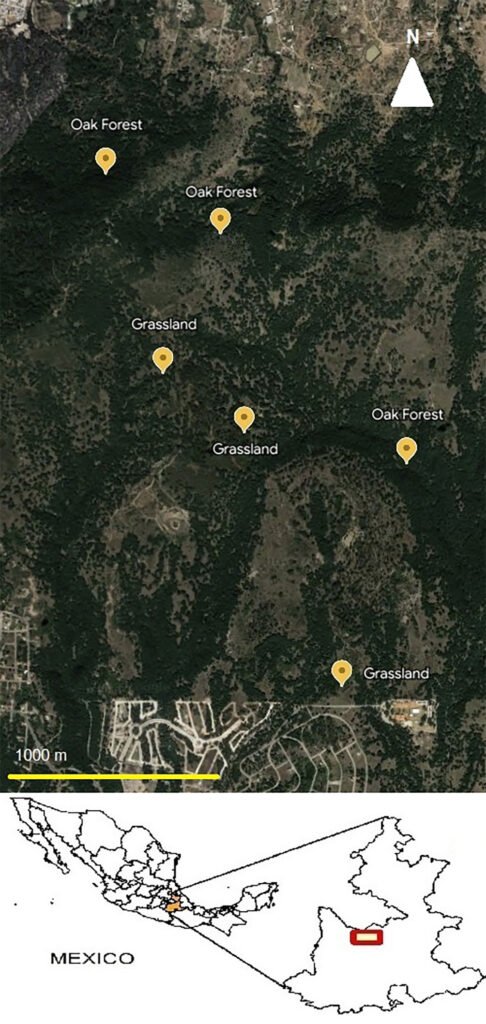
Figure 1. Localization of the transects used in the study zone (mapped by Luna F.).
We chose numerical dominance to assess the dominance hierarchy of the ants —i.e., ordering of ant species based on their numerical or behavioral dominance by vegetation types (Andersen, 1992; Cerdá et al., 1997; Stuble et al., 2017), ant species and bait type. As numerical and behavioral dominance are highly correlated, this method has been well-established and documented in the ant literature (Dáttilo et al., 2014; Dejean & Corbara, 2003; Parr, 2008; Parr & Gibb, 2012; Santini et al., 2007). This method indicates which species are consistently present at the baits, and which ones dominate the baits numerically and thus monopolize them (Parr, 2008). We represented numerical dominance using the numerical dominance index (DI) for each morphospecies calculated by the formula: DI = (Di)/(Di + Si), where, Di is the number of baits monopolized by the species of ant i, and Si is the number of baits that the species of ant i used but did not monopolize. Baits were considered to be monopolized when more than 5 individuals (workers and/or soldiers) of the same morphospecies were using the resource without the presence of other morphospecies. This measure (more than 5 individuals) takes into consideration that in temperate climates, ants are less abundant, and recruitment is considered weaker than in tropical environments where the index has been widely more (Santini et al., 2007). Therefore, dominant morphospecies are those that find and monopolize a larger proportion of the food resources in a given environment. The value of the index ranges from 0 (completely submissive species) to 1 (totally dominant species) and is similar to the “monopolization index” used in other studies (Dáttilo et al., 2014; Fellers, 1987; Parr & Gibb, 2012; Santini, et al., 2007). In this study, ant species with a DI lower than 0.5 were classified as submissive.
The arrival times of the ants were compared using survival curves using the survival package of the R Software program. The one-hour and 2-hour baits were used to calculate the ID of the ant species. To compare the ID between vegetation and bait types, t-tests were performed. To compare the DI between ant species, a one-way ANOVA test was performed after applying a square root transformation to meet the normality requirements. These analyses were performed using the program STATVIEW 5.0 (Abacus Concepts Inc., 1996).
Results
Fourteen morphospecies of ants were recorded in this study belonging to 11 ant genera (Table 1). For the analysis of the results from the nests and the baits placed near them, 2 morphospecies of Pheidole were identified. However, due to the low number of records for each morphospecies across different vegetation types, Pheidole sp. 1 and Pheidole sp. 2 were combined for the analysis. In the oak forest, 2 out of 19 records corresponded to Pheidole sp. 1, and in the grassland, 4 out of 15 records corresponded to Pheidole sp. 2. Since Pheidole species often share similar feeding habits and are functionally similar (Agosti, 2000; Andersen, 2000), these 2 morphospecies were grouped to increase statistical power, and the analysis was conducted at the genus level. In contrast, another Pheidole species, Pheidole sp. 3, was recorded in the baits used to determine the DI and was analyzed independently due to its different context of occurrence. The rest of the analyses were conducted at the species level.
In the case of the nests, we found 9 morphospecies distributed among 34 nests, 19 of these were located in the oak forest and 18 in the induced grassland (Table 2). No significant differences were observed in the types of food elements those individual ants transported to their nest when comparing different vegetation types (x2 = 2.13, df = 2, p = 0.34). In the oak forest considering all ant species, there were no significant differences in the number of individuals carrying elements from different categories (x2 = 0.03, df = 1, p = 0.86) (Fig. 2). Of the genera of ants observed carrying food elements to their nests, only 2 genera were observed with a single type of food resource: Prenolepis imparis (Say) only carried arthropods and Dorymyrmex insanus (Buckley) only seeds.
Table 1
List of ant species recorded in this study in “Flor del Bosque” State Park in Puebla, Mexico.
| Formicidae/Subfamily/Ant species | |||
| Formicidae Latreille, 1809 | |||
| Dolichoderinae Forel, 1878 | |||
| Leptomyrmecini Emery, 1913 | |||
| Dorymyrmex Mayr, 1866 | |||
| Dorymyrmex insanus (Buckley, 1866) | |||
| Linepithema Mayr, 1866 | |||
| Linepithema dispertitum (Forel, 1885) | |||
| Dorylinae Leach, 1815 | |||
| Dorylini Ashmead, 1905 | |||
| Labidus Jurine, 1807 | |||
| Labidus coecus Latreille, 1802 | |||
| Formicinae Latreille, 1809 | |||
| Camponotini Forel, 1878 | |||
| Camponotus Mayr,1861 | |||
| Camponotus rubrithorax Forel, 1899 | |||
| Lasiini Ashmead, 1905 | |||
| Nylanderia Emery, 1906 | |||
| Nylanderia austroccidua Trager, 1984 | |||
| Prenolepis Mayr, 1861 | |||
| Prenolepis imparis Say, 1836 | |||
| Myrmicinae Lepeletier, 1835 | |||
| Attini Smith, 1858 | |||
| Pheidole Westwood, 1839 | |||
| Pheidole sp. 1 | |||
| Pheidole sp. 2 | |||
| Pheidole sp. 3 | |||
| Crematogastrini Forel, 1893 | |||
| Temnothorax Mayr, 1861 | |||
| Temnothorax augusti Baroni Urbani, 1978 | |||
| Pogonomyrmecini Ward, Brady, Fisher & Schultz, 2014 | |||
| Pogonomyrmex Mayr, 1868 | |||
| Pogonomyrmex barbatus (Smith, F., 1858) | |||
| Solenopsidini Forel, 1893 | |||
| Monomorium Mayr, 1855 | |||
| Monomorium ebenium Forel, 1891 | |||
| Pseudomyrmecinae, Smith, M.R., 1952 | |||
| Pseudomyrmecini Smith, 1952 | |||
| Pseudomyrmex Lund, 1831 | |||
| Pseudomyrmex pallidus Smith, F., 1855 |
Conversely, ants of the genus Pheidole spp. carried a greater quantity of plant elements compared to the other categories (x2 = 10.4, df = 2, p = 0.006); only 2 of these individuals were observed carrying arthropods, and none were recorded carrying seeds. No significant differences were found for D. insanus (x2 = 2, df = 2, p = 0.37), P. barbatus (x2 = 1.6, df = 2, p = 0.450), nor P. imparis (x2 = 4, df = 2, p = 0.135) among the 3 food categories. Only P. barbatus carried all 3 types of food (Fig. 2).
Considering all ant species, there were no significant differences in the number of individuals carrying different food elements in the induced grassland (x2 = 0.66, df = 1, p = 0.41, Fig. 2). Among the observed ant species that transported food types to their nests, P. barbatus was found to carry both plant elements and seeds. Additionally, Dorymyrmex insanus and Pheidole spp. were observed carrying food from all 3 categories (Fig. 2). Pheidole spp. (x2 = 10.33, df = 2, p = 0.006) and P. barbatus (x2 < 30.33, df = 2, p < 0.001), carried a higher number of plant elements. For D. insanus no significant differences were found in the number of elements of each type (x2 < 3.77, df = 2, p = 0.15) (Fig. 2).
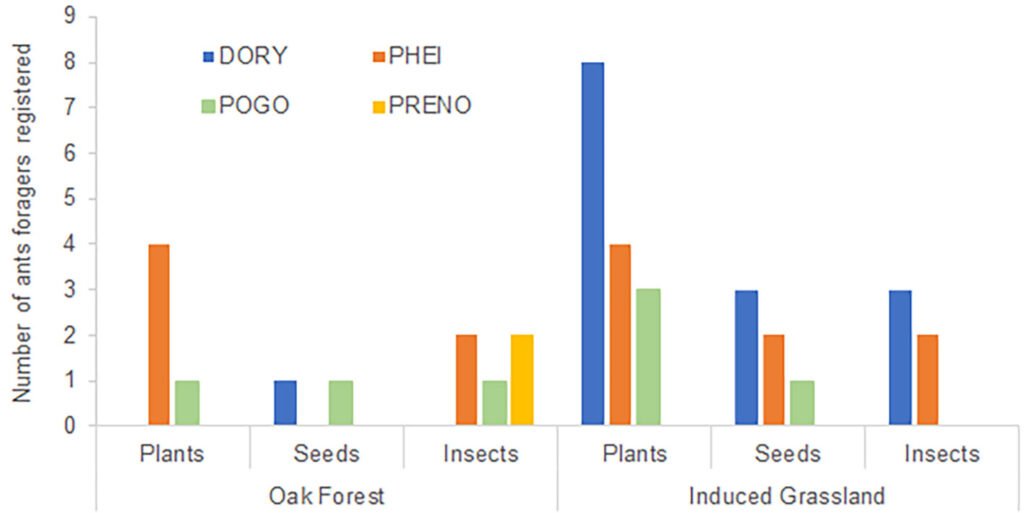
Figure 2. Number of individuals of the different ant species recorded carrying any food resource to their nest. DORY: Dorymyrmex insanus, PHEI: Pheidole spp., POGO: Pogonomyrmex barbatus, PREN: Prenolepis impairs.
In the induced grassland, 5 ant species were attracted to both types of baits, and 1 species was found in the tuna baits. In the oak area, 5 ant species were observed in both types of baits, and 1 species was attracted to the honey bait (Fig. 3). The probability of presence of a forager during a given minute of the observation period depended on the vegetation type (x2= 8.73, df = 1, p = 0.03), the ant species (x2= 59.64, df = 7, p < 0.001), the interaction between the ant species and the bait (x2= 57.96, df = 8, p < 0.001), and the interaction between the vegetation type, the ant species, and the bait (x2= 26.12, df = 7, p < 0.001). The Tukey contrast test for the vegetation*species*bait interaction showed (Z > 3.83, p < 0.042) that the presence of Linepithema dispertitum (Forel) on the honey bait, in the oak forest, was more likely than the presence of Pheidole spp. on either the tuna or honey bait in the oak forest, Pheidole spp. on the honey bait in the induced grassland, and P. impairs on the honey bait in the oak forest (Fig. 3). The probability of Camponothus rubrithorax (Forel) being present on the honey bait in the induced grassland, was greater than that of D. insanus in either the tuna or honey bait in the induced grassland, Pheidole spp. on either the tuna or honey bait in the oak forest, as well as its presence in the honey bait in the induced grassland and P. imparis in the honey bait in the oak forest (Fig. 3). The probability that P. barbatus was present on the honey bait in the induced grassland, was greater than the presence of Pheidole spp. in either the tuna or honey bait and P. imparis in the honey bait in the oak forest (Fig. 3). Finally, the presence of Pheidole spp. on the tuna bait was more likely than its presence on the honey bait in the induced grassland (Fig. 3).
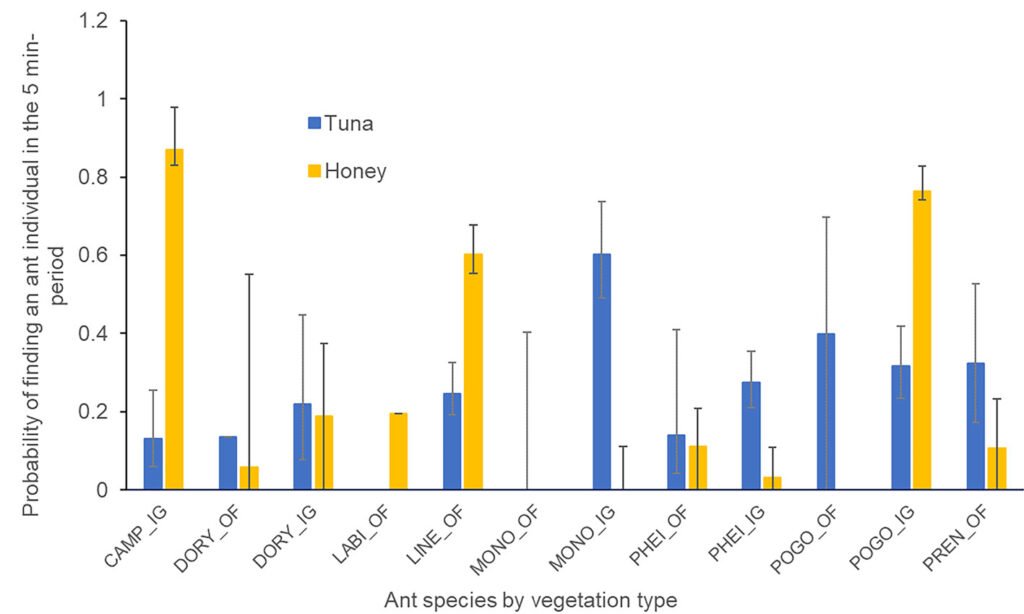
Figure 3. Probability of finding an ant forager on the honey or tuna baits at the nest of the different ant species registered in the Oak Forest (OF) and the Induced Grassland (IG) in the 5-minute period. CAMPO: Camponotus rubrithorax, DORY: Dorymyrmex insanus, LABI: Labidus coecus, LINE: Linepithema dispertitum, MONO: Monomorium ebenium, PHEI: Pheidole spp., POGO: Pogonomyrmex barbatus, PREN: Prenolepis imparis.

Figure 4. Survival curves depicting the arrival times of ant foragers on the baits (tuna or honey) during a one-hour observation.
No significant differences were found in the arrival times among ant species.
No significant differences were found in the arrival times between the vegetation types (x2 = 0.4, df = 1, p = 0.5). Moreover, no significant differences were found in the arrival times among the different ant species (x2 = 7.6, df = 7, p = 0.4) or the type of bait (x2 = 0.01, df = 1, p = 0.9; Fig. 4).
In the dominance index baits 12 ant morphospecies were recorded and different ant morphospecies were recorded in each of the vegetation types (Table 3). When comparing the average dominance index (DI) by vegetation type (mean ± SE, n; oak forest DI = 0.372 ± 0.071, 33; induced grassland DI = 0.251 ± 0.052, 44), was not statistically different (t= 1.091; df = 75; p = 0.2787), which would suggest a submissive behavior in both types of vegetation. Average dominance index by ant species indicates that M. ebenium, P. imparis and Pheidole sp. 3 behave as submissive in both vegetation types (t = 0.590, df = 20, p = 0.5617; t = -0.237, df = 10, p = 0.8171; t = -0.315, df = 11, p = 0.7590, respectively) (Table 4). It was not possible to compare the DI between vegetation types for Temnothorax sp., T. augusti, C. rubrithorax, N. austroccidua, L. dispertitum, D. insanus or P. pallidus, because they were only present in one of the vegetation types (Table 4).
The average dominance index for each ant species was low suggesting that all of them displayed submissive behaviors (F8,68 = 1.949, p = 0.06). Nylanderia austroccidua and L. dispertitum had the highest dominance indices, although it should be noted that N. austroccidua only had one record and L. dispertitum had 3 records. Additionally, Monomorium ebenium showed a tendency to behave as dominant (Table 4).
When comparing the mean dominance index by bait type, no significant differences were found (t = 1.023, df = 110, p = 0.3088; mean ± SE, n; tuna = 0.382 ± 0.060, 55; honey = 0.295 ± 0.053, 57). This indicates that ants exhibited submissive behavior in both types of bait.
According to the average dominance index per ant species in relation to the bait type, Pheidole sp. 3, P. imparis, M. ebeninum, C. rubrithorax, and D. insanus exhibited a submissive behavior in both types of baits (t = 0.888, df = 13, p = 0.3907; t= 0.988, df = 15, p = 0.3388; t = 0.189, df = 32, p = 0.8513; t = -0.918, df = 24, p = 0.3675, t = 2.390, df = 5, p = 0.0624, respectively) (Table 5) and L. dispertitum exhibited a dominant behavior in both types of baits (t = 0.421, df = 3, p = 0.7021).
Temnothorax sp. and P. pallidus were not compared statistically due to the low incidence registered; nonetheless, they presented a submissive behavior index in both types of bait. Neither T. augusti nor N. austroccidua were included in this analysis, as they were only present on the honey bait. In none of the species was there a difference in behavior between the baits.
Table 2
Number of nests found in the oak forest and the induced grassland.
| Species | Oak forest | Induced grassland |
| Camponotus rubrithorax | 0 | 3 |
| Dorymyrmex insanus | 4 | 6 |
| Labidus coecus | 1 | 0 |
| Linepithema dispertitum | 2 | 0 |
| Pheidole spp. | 7 | 6 |
| Pogonomyrmex barbatus | 1 | 2 |
| Prenolepis imparis | 3 | 0 |
| Monomorium ebenium | 1 | 1 |
Discussion
This study found that certain ant species (e.g., C. rubrithorax, L. dispertitum, P. barbatus) exhibited a preference for honey within specific vegetation types when compared to the rest of the ant community. The compensation hypothesis (Davidson, 2005; Kaspari & Yanoviak, 2001) predicts that the attractiveness of a nutrient to an organism is higher the more limiting it is. It is possible that this result is related to the fact that sugar is generally less available on the ground strata than protein (Kaspari & Yanoviak, 2001; Kaspari et al., 2012).
However, when considering the overall community level, no noticeable food preferences were found between the ant communities in the oak forest and the induced grassland vegetation. This lack of preference was evident both in the items carried to their nests and the resources provided on the baits. There were no significant differences in arrival times at the baits between vegetation types. This similarity can be attributed to ants experiencing soil-level heterogeneity similarly in both oak forests and induced grasslands (see below).
The ant communities in both habitats were composed of ant species displaying submissive behavior. This characteristic could be attributed to stress factors such as low temperatures in the oak forest and disturbances in the induced grassland, which potentially reduce ant competition. In the oak forest, low temperatures may favor the presence of cold climate specialists that can forage at low temperatures without the need to display a dominant behavior. In the induced grassland, disturbances may favor the presence of generalist species that take advantage of the absence of dominant species such as the dominant Dolichoderinae (see below). This study emphasizes the significance of ant species’ response to their environment and their adaptability in dealing with disturbances.
Our findings do not provide support for vegetation-scale disparities in food preferences, first and second hypotheses, which proposed greater differences in the food items being carried to the nest in the oak forest and reduced food preferences in the induced grassland. There were no differences between vegetation types in the number of seeds, plants, or arthropods taken by the ant foragers to their nests. Similarly, there was not a preference for a specific food bait (honey or tuna) between the oak forest and induced grassland ant communities. These findings are consistent with previous studies conducted by Radnan et al. (2018) and Castillo-Guevara et al. (2019), which did not identify differences in food preferences or foraging strategies between natural and disturbed vegetation at the community level. However, our observations did reveal that certain ant species exhibited preferences for specific types of food, within particular vegetation types, in accordance with the first hypothesis and second hypothesis but at the species level, which proposed an influence of ant species on food preferences. The specific preferences of these ant species are discussed in detail below.
Table 3
Visit frequency (i.e., number of foragers) per species in each vegetation type and functional groups they belong to (Andersen, 2000). GM = Generalized Myrmicinae, CCS = cold climate specialist, SC = subordinate Camponotini, TCS = tropical climate specialist, O = opportunist.
| Subfamily | Specie | Funtional group | Oak forest | Induced grassland |
| Myrmicinae | Monomorium ebenium | GM | 753 | 167 |
| Myrmicinae | Pheidole sp. 3 | GM | 14 | 236 |
| Myrmicinae | Temnothorax sp. | CCS | 5 | 0 |
| Myrmicinae | Temnothorax augusti | CCS | 0 | 1 |
| Formicinae | Prenolepis imparis | CCS | 289 | 11 |
| Formicinae | Camponotus rubrithorax | SC | 0 | 351 |
| Formicinae | Nylanderia austroccidua | TCS | 0 | 30 |
| Dolichoderinae | Linepithema dispertitum | CCS | 190 | 0 |
| Dolichoderinae | Dorymyrmex insanus | O | 0 | 26 |
| Pseudomyrmecinae | Pseudomyrmex pallidus | TCS | 0 | 10 |
| Total | 1,251 | 832 |
Table 4
Average numerical dominance index (DI) (mean ± SE, n) per ant morphospecies. (-) Unregistered species.
| Specie | Oak forest | Induced grassland | ID |
| Monomorium ebenium | 0.4 ± 0.1, 13 | 0.3 ± 0.1, 9 | 0.4 ± 0.0, 22 |
| Pheidole sp.3 | 0.2 ± 0.2, 4 | 0.2 ± 0.1, 9 | 0.2 ± 0.1, 13 |
| Temnothorax sp. | 0 ± 0, 3 | – | 0 ± 0, 3 |
| Temnothorax augusti | – | 0, 1 | 0 ± 0, 1 |
| Prenolepis imparis | 0.3 ± 0.1, 10 | 0.5 ± 0.5, 2 | 0.3 ± 0.1, 12 |
| Camponotus rubrithorax | – | 0.1 ± 0.0, 15 | 0.1 ± 0.0, 15 |
| Nylanderia austroccidua | – | 1, 1 | 1, 1 |
| Linepithema dispertitum | 0.7 ± 0.1, 3 | – | 0.7 ± 0.1, 3 |
| Dorymyrmex insanus | – | 0.2 ± 0.1, 4 | 0.3 ± 0.1, 4 |
| Pseudomyrmex pallidus | – | 0 ± 0, 3 | 0 ± 0, 3 |
Pheidole spp., in the induced grassland, were observed carrying a greater quantity of plant elements to their nests in both vegetation types. However, it exhibited a preference for the tuna bait in induced grassland, possibly indicating a supplementary dietary preference. Given the extensive diversity within the Pheidole genus, which encompasses 900 species described worldwide (Wilson, 2003), it is not feasible to categorize them based on a specific food habit and our results suggest that it is omnivorous (Table 6). Nevertheless, these results should be interpreted with caution due to the grouping of species.
Camponotus rubrithorax and P. barbatus, in the induced grassland, as well as L. dispertitum, in the oak forest, were more frequently observed foraging the honey bait compared to other ant species. Camponotus (Mayr) is a genus known for its nectarivorous habits and consumption of other sweet secretions, such as honeydew which coincides with its preference for honey found in the present study (Nettimi & Iyer, 2015). In this study, L. dispertitum predominantly consumed honey, although it is a generalist forager species capable of consuming other types of food as well (Table 5), in the study site it is found exclusively in the oak forest (Cuautle et al., 2016).
Despite Pogonomyrmex (Mayr) has been recognized as a granivorous genus (Pirk & López-de Casenave, 2014), this study provided new insights into the foraging preferences of P. barbatus, revealing a notable inclination towards carbohydrates and plant elements within the induced grassland. Moreover, we even registered individuals transporting arthropods to their nests. These findings (Table 5) strongly indicate that certain species within the Pogonomyrmex genus exhibit a generalist foraging behavior. Other species such as D. insanus, which used resources more intensively in the induced grassland, showed no preference for any of the baits or items taken to the nest, which coincides with the generalist forager behavior, observed in open and disturbed habitats by Cuezzo and Guerrero (2012).
Table 5
Average numerical dominance index (DI) (mean ± SE, n) per ant species in both bait types (tuna, honey). (-) Unregistered species.
| Species | Tuna | Honey |
| Monomorium ebenium | 0.4 ± 0.1, 18 | 0.4 ± 0.1, 16 |
| Pheidole sp.3 | 0.4 ± 0.2, 7 | 0.1 ± 0.1, 8 |
| Temnothorax sp. | 0, 1 | 0 ± 0, 2 |
| Temnothorax augusti | – | 0, 1 |
| Prenolepis imparis | 0.5 ± 0.1, 7 | 0.3 ± 0.1, 10 |
| Camponotus rubrithorax | 0.1 ± 0.0, 14 | 0.2 ± 0.0, 12 |
| Nylanderia austroccidua | – | 1, 1 |
| Linepithema dispertitum | 0.7 ± 0.2, 2 | 0.6 ± 0.3, 3 |
| Dorymirmex insanus | 0.6 ± 0.3, 3 | 0 ± 0, 4 |
| Pseudomyrmex pallidus | 0 ± 0, 3 | 0, 1 |
Table 6
The biology and ecology of the ant genera found in this study, in the Flor del Bosque State Park (Agosti et al., 2000; AntWiki, n.d.).
| Genera | Microhabitat | Food habits |
| Camponotus | Ground nesting, decaying wood and in trees | Generalist foragers |
| Dorymyrmex | ————– | Generalist foragers |
| Linepithema | ————– | Generalist foragers |
| Labidus | Epigeous,bivouacs | Predators (Army ants) |
| Monomorium | ————– | Generalized foragers, harvesters |
| Nylanderia | Nest in leaf litter, soil, or in rotten wood | Generalist foragers |
| Pheidole | Soil or decaying wood | Granivores or omnivores |
| Pogonomyrmex | Ground nesting | Generalist foragers and granivores |
| Prenolepis | ————– | Generalist predators |
| Pseudomyrmex | Mostly arboreal (nesters and foragers), few epigaeic | Generalized predators, visit extrafloral nectaries |
| Temnothorax | Nesting in ground, and under stones, in wood, and in trees | Generalized foragers and parasites |
The previous results (e.g., C. rubrithorax, Pheidole spp., D. insanus) support the reported food habits of specific ant genera. Nonetheless, it is also possible to interpret these results as ants taking nutrients from the baits that are not currently available or that are limited within their community to supplement their diet (Compensation hypothesis) (Davidson, 2005; Kaspari & Yanoviak, 2001). In this study, significant differences in ant species presence on baits were primarily associated with the presence of ant species on honey baits. This finding aligns with the compensation hypothesis (Davidson, 2005; Kaspari & Yanoviak, 2001), which posits that the utility of a resource remains constant across species and varies solely with availability. The hypothesis predicts a singular limiting resource that is locally in shortest supply. Consequently, habitats with relatively high protein availability should attract ants more inclined towards carbohydrates, and vice versa. Previous research has demonstrated that litter ant communities are limited by carbohydrates, whereas ant arboreal communities face protein limitations in tropical regions (Kaspari & Yanoviak, 2001; Kaspari et al., 2012). Although this study was conducted in a temperate environment, the results might be related to the usual scarcity of sugar in the ground strata compared to protein.
Our results do not align with the second hypothesis, which speculated faster arrival times in the induced grassland. We did not find significant differences in arrival times among vegetation types, ant species, or bait types. Ants could be experiencing similar heterogeneities at the soil level between the oak forest and induced grassland. While it is expected that the presence of more litter in the oak forest could hinder ant movement, within the induced grassland, the vegetation morphology itself (long grass species) could be interfering with ant movement. For instance, Hernández-Flores et al. (2016) observed that the foraging performance of P. barbatus was reduced due to the presence of herbaceous vegetation in plots where regeneration after grazing was permitted.
Dominance hierarchy. Disturbance typically favors the presence of generalist and opportunistic species, hence, we expected to find a lower dominance index within the induced grassland (third hypothesis). Nonetheless, we did not find enough evidence to support this hypothesis as we registered ant communities consisted of submissive species in both habitats. Additionally, the ants did not show a tendency to dominate a specific resource, neither at the bait level nor at the species level, suggesting a lack of food preferences. These results are consistent with the findings of Castillo-Guevara et al. (2019), who registered no significant differences in dominance indices between a native oak forest and an altered agricultural land. Moreover, the authors reported intermediate to low values of dominance within the ant communities of each vegetation type. Our findings could be attributed to the presence of other factors that could have overshadowed the role of competition in shaping the organization of ant communities. For example, a review by Parr and Gibb (2012), which encompassed data from 3 continents, indicated that the trade-off between discovery and dominance occurs primarily when parasitoids are present. In environments without parasitoids, species with high discovery abilities tend to also be dominant (Parr & Gibb, 2012). While our study did not specifically assess the discovery-dominance trade-off, the observed lack of differences in arrival times and similar dominance values among the ants suggest that dominance was not a prominent factor in our study sites. It is possible that factors such as low temperatures in the oak forest, or disturbance in the induced grassland, may have played a role in relaxing dominance. According to Andersen (2000), in disturbed vegetation like the induced grassland, it is expected to observe the presence of subdominant ant species that exploit the absence of dominant species from the native vegetation, such as dominant Dolichoderinae. The findings obtained during this study align with this prediction and provide support for it. Notwithstanding, the absence of ants with high dominance indices in the studied communities does not necessarily indicate a complete lack of dominance hierarchy. In each community, the ants can still be ordered based on their DI. For example, in the oak forest, L. dispertitum had the highest DI value (0.7 DI), M. ebenium had an intermediate value (0.4 DI) and Temnothorax spp. had the lowest value (0.0 DI).
Land use change did not seem to influence food preferences or foraging strategies at the community level. However, we observed an effect at species level, indicating that individual ant species exhibited specific food preferences. Carbohydrates could be the limiting resource in the oak forest and the induced grassland litter ant communities, as some ant species showed preference for honey baits. Although competition is typically considered a key factor in understanding food preferences among ants, it is noteworthy that both, the natural oak forest and induced grassland, were predominantly populated by submissive ant species. Therefore, it appears that other factors instead of competition may be playing a role in shaping food preferences within these communities. In conclusion, our study reveals that ant species may exhibit preferences for specific foods, which could be limited in their environment. The ability of ants to respond to available resources enables them to optimize their nutrient intake, as well as adapt and persist under variable conditions, including disturbances. Understanding the distinct dietary preferences and foraging strategies of ant species within functional groups will provide valuable insights into their ecological roles and potential impacts on ecosystem dynamics. Such investigations would enhance our ability to predict the responses of ants to diverse forms of disturbances in an anthropized world.
Acknowledgements
We appreciate the assistance provided by the authorities of “Flor del Bosque” State Park, coordinator Enrique Martínez Romero (M.S.) and director Mario Alberto Romero Guzmán (MVZ). We would also like to thank Florencio Luna Castellanos for his support with the fieldwork. This study was financed by Consejo Nacional de Humanidades Ciencias y Tecnologías (Conahcyt) as part of a grant awarded to Mariana Cuautle (223033).
References
Abacus Concepts Inc. (1996). Abacus Concepts, Stat View Reference. Berkeley, California.
Agosti, D., Majer, J. D., Alonso, L. E., & Schultz, T. R. (2000). Ants: standard methods for measuring and monitoring bio-
diversity. Washington D.C.: Smithsonian Institution Press.
Andersen, A. (2000). A global ecology of rainforest ants: functional groups in relation to environmental stress and disturbance. In D. Agosti, J. D. Majer, L. E. Alonso, & T. R. Schultz (Eds.), Ants: standard methods for measuring and monitoring biodiversity (pp. 25–34). Washington D.C.: Smithsonian Institution Press.
Andersen, A.N. (1992). Regulation of “momentary” diversity by dominant species in exceptionally rich ant communities of the Australian seasonal tropics. The American Naturalist, 40, 401–420. https://www.journals.uchicago.edu/doi/abs/10.
1086/285419
AntWiki (n.d.). Nylanderia. AntWiki. Consulted 8/20/2024
Arnan, X., Cerdá, X., & Retana, J. (2012). Distinctive life traits and distribution along environmental gradients of dominant and subordinate Mediterranean ant species. Oecologia, 170, 489–500. https://doi.org/10.1007/s00442-012-2315-y
Castillo-Guevara, C., Cuautle, M., Lara, C., & Juárez-Juárez, B. (2019). Effect of agricultural land-use change on ant dominance hierarchy and food preferences in a temperate oak forest. PeerJ, 7, e6255. https://peerj.com/articles/6255/
Cerdá, X., Arnan, X., & Retana, J. (2013). Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology? Myrmecological News, 18, 131–147. https://doi.org/10.25849/myrmecol.news_018:131
Cerdá, X., Retana, J., & Cros S. (1997). Thermal disruption of transitive hierarchies in Mediterranean ant communities. Journal of Animal Ecology, 66, 363–374.
Costes-Quijano, R., Meza, A. R., Macías-Juárez, A., Berriel-Mastreta, C. A., Cortés-Atilano, B., Martínez-Romero, L. E. et al. (2006). Plan de manejo Parque Ecológico Recreativo General Lázaro Cárdenas “Flor del Bosque”. [Management Plan of the recreative ecological Park “General Lázaro Cárdenas “Flor del Bosque”]. Ciudad de México: Gobierno del Estado de Puebla/ Secretaría de Medio Ambiente y Recursos Naturales.
Cuautle, M., Vergara, C., & Badano, E. (2016). Comparison of ant community diversity and functional group composition associated to land use change in a seasonally dry oak forest. Neotropical Entomology, 45, 170–9. https://doi.org/
10.1155/2012/516058
Cuezzo, F., & Guerrero, R. J. (2012). The Ant Genus Dorymyrmex Mayr (Hymenoptera: Formicidae: Dolicho-
derinae) in Colombia. Psyche, 51605, 1–24. https://doi.org/
10.1155/2012/516058
Dáttilo, W., Díaz-Castelazo, C., & Rico-Gray, V. (2014). Ant dominance hierarchy determines the nested pattern in ant-plant networks. Biological Journal of the Linnean Society, 113, 405–414. https://doi.org/10.1111/bij.12350.
Davidson, D. W. (2005). Ecological stoichiometry of ants in a New World rain forest. Oecologia, 142, 221–231. https://doi.org/10.1007/s00442-004-1722-0
Dejean, A., & Corbara, B. (2003). A review of mosaics of dominant ants in rainforests and plantations. In Y. Basset, V. Novotny, S. E. Miller, & R. L. Kitching (Eds.), Arthropods of tropical forests: spatio-temporal dynamics and resource use in the canopy (pp 341–347). Cambridge: Cambridge University Press.
Ellis, E. C., Goldewijk, K. K., Siebert S., Lightman, D., & Ramankutty, N. (2010). Anthropogenic transformation of
the biomes, 1700 to 2000. Global Ecology and Biogeo-
graphy, 19, 589–606. https://doi.org/10.1111/j.1466-8238.20
10.00540.x
Fellers, J. H. (1987). Interference and exploitation in a guild of Woodland ants. Ecology, 68, 1466–1478. https://doi.org/
10.2307/1939230
Fellers, J. H. (1989). Daily and seasonal activity in woodland
ants. Oecologia, 78, 69–76. https://doi.org/10.1007/BF0037
7199
Foley, J. A., DeFries, R., Asner, G. P., Barford C., Bonan, G., Carpenter, S. R. et al. (2005). Global consequences of land use. Science, 309, 570–574. https://doi/10.1126/science.1111772
Hoffmann, B. D., & Andersen, A. N. (2003). Responses of ants to disturbance in Australia, with particular reference to functional groups. Austral Ecologyl, 28, 444–464. –https://doi.org/10.1046/j.1442-9993.2003.01301.x
Houdria, M., Salas-López, A., Orivel. J., Bluthgen, N., & Menzel, F. (2015). Dietary and temporal niche differentiation in tropical ants – can they explain local ant coexistence? Biotropica, 47,208–217. https://doi.org/10.1111/btp.12184
Hernández-Flores, J., Osorio-Beristain. M., & Martínez-Garza. C. (2016). Ant foraging as an indicator of tropical dry forest restoration. Environmental Entomology, 45, 991–994. https://doi.org/10.1093/ee/nvw054
Kaspari, M., Donoso, D., Lucas, J. A., Zumbusch, T., & Kay, A. D. (2012). Using nutritional ecology to predict community structure: a field test in Neotropical ants. Ecosphere, 3,93. https://doi.org/10.1890/ES12-00136.1
Kaspari, M., & Yanoviak, S. P. (2001). Bait use in tropical litter and canopy ants-evidence of differences in nutrient limitation. Biotropica, 33,207–211. https://doi.org/10.1646/
0006-3606(2001)033[0207:BUITLA]2.0.CO;2
Łaska, G. (2001). The disturbance and vegetation dynamics: a review and an alternative framework. Plant Ecology, 157, 77–99. https://doi.org/10.1023/A:1013760320805
Lynch, J. F., Balinsky, E. C., & Vail, S. G. (1980). Foraging patterns in three sympatric forest ant species, Prenolepis imparis, Paratrechina melanderi and Aphaenogaster rudis (Hymenoptera: Formicidae). Ecological Entomology, 5,353–371. https://doi.org/10.1111/j.1365-2311.1980.tb01160.x
Mackay, W., & Mackay, E. (1989). Clave de los géneros de hormigas en México (Hymenoptera: Formicidae). El Paso, Texas: The University of Texas.
Nettimi, R. P., & Iyer, P. (2015). Patch fidelity in Camponotus compressus ants foraging on honeydew secreted by treehoppers. Current Science, 109, 362–366.
Parr, C. L. (2008). Dominant ants can control assemblage species richness in a South Africa savanna. Journal of Animal Ecology, 77, 1191–1198. https://doi.org/10.1111/j.13
65-2656.2008.01450.x
Parr, L., & Gibb, H. (2012). The discovery-dominance trade-off
is the exception, rather than the rule Journal of Animal
Ecology, 81, 233–241. https://doi.org/10.1111/j.1365-2656.
2011.01899.x
Pirk, G. I., & López-de Casenave, J. (2014). Effect of harvester ants of the genus Pogonomyrmex on the soil seed bank around their nests in the central Monte desert, Argentina. Ecological Entomology, 39, 610–619. https://doi.org/10.1111/een.12140
R Core Team (2022). R: a language and environment for statistical computing. Vienna, Austria: R Foundation
for Statistical Computing. https://www.R-project.org/
Radnan, G. N., Gibb, H., & Eldridge, D. J. (2018). Soil surface complexity has a larger effect on food exploitation by ants than a change from grassland to shrubland. Ecological Entomology, 43, 379–388. https://doi.org/10.1111/een.12510
Rico-Gray, V., & Oliveira, P. S. (2007). The ecology and evolution of ant-plant interactions. Chicago: University of Chicago Press.
Sala, O. E., Chapin, F. S., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo. R. et al. (2000). Global biodiversity scenarios for the year 2100. Science, 287,1770–1774. https://doi.org/10.1126/science.287.5459.17
Santini, G., Tucci, L., Ottonetti, L., & Frizzi, L. (2007). Competition trade-offs in the organisation of a Medite-
rranean ant assemblage. Ecological Entomology, 32,
319–326. https://doi.org/10.1111/j.1365-2311.2007.00882.x
Savolainen, R., Vepsäläinen, K., & Wuorenrinne, H. (1989). Ant assemblages in the taiga biome: testing the role of territorial wood ants. Oecologia, 81, 481–486. https://doi.org/10.1007/BF00378955