Revisión de Hermenia con descripciones de cinco nuevas especies y un género nuevo (Polychaeta: Polynoidae)
Patricia Salazar-Silva a, *, Sergio I. Salazar-Vallejo b
a Tecnológico Nacional de México, Instituto Tecnológico de Bahía de Banderas, Crucero a Punta de Mita, 63734 Bahía de Banderas, Nayarit, Mexico
b El Colegio de la Frontera Sur, Unidad Chetumal, Depto. Sistemática y Ecología Acuática, Av. Centenario Km 5.5, 77019 Chetumal, Quintana Roo, Mexico
*Corresponding author: salazarsilva01@yahoo.com (P. Salazar-Silva)
Received: 04 March 2025; accepted: 23 July 2025
http://zoobank.org/urn:lsid:zoobank.org:pub:08AE4922-3010-4327-B8AA-9753DD0AE1D1
Abstract
Hermenia Grube & Örsted (in Grube, 1856) contains 3 described species, with H. verruculosa Grube & Örsted (in Grube, 1856) as its type species and distributed in the Grand Caribbean. H. acantholepis (Grube, 1876) from the Philippines, and H. neoverruculosa Pettibone, 1975 from the Cargados Carajos, Republic of Mauritius. After the revision of type and non-type specimens, differences were observed in body shape, pigmentation pattern, prostomium, size of elytra, dorsal tubercles, and ventral papillae. Type material for the 3 species in the genus was revised, and Hermenia is redefined, its species are redescribed, and 4 species are described: H. chuarae sp. nov. from Indonesia, H. mezianei sp. nov. from Vietnam, H. treadwelli sp. nov. from the Grand Caribbean, and H. wehei sp. nov. from the Arabian Sea. On the other hand, Lepidonotus hermenioides Amoureux, 1974, described from Madagascar, resembles Hermenia species by having papillate integument, but differs by having elytra with long fimbriae. Parahermenia gen. nov. is proposed for including it, and Parahermenia piotrowskiae sp. nov. from Philippines. Identification keys for all species of Hermenia and Parahermenia were included.
Keywords: Caribbean; Lepidonotus; Parahermenia; Scaleworms; Taxonomy
Resumen
Hermenia Grube et Örsted (in Grube, 1856) contiene 3 especies descritas, con H. verruculosa Grube et Örsted (in Grube, 1856) como la especie tipo y distribuida en el Gran Caribe, H. acantholepis (Grube, 1876) de Filipinas y H. neoverruculosa Pettibone, 1975 de Cargados Carajos, República de Mauricio. Después de la revisión del material tipo y de ejemplares no tipo, se observaron diferencias en la forma del cuerpo, en el patrón de pigmentación, prostomium, tamaño de los élitros, tubérculos dorsales y papilas ventrales. El material tipo para las 3 especies fue revisado y Hermenia es redefinida, sus especies son redescritas y se describen 4 nuevas: H. chuarae sp. nov. de Indonesia, H. mezianei sp. nov. de Vietnam, H. treadwelli sp. nov. del Gran Caribe y H. wehei sp. nov. del mar Arábigo. Por otro lado, Lepidonotus hermenioides, descrita de Madagascar, es similar a las especies de Hermenia por tener integumento papilado, pero difiere por tener élitros con fimbria larga. Parahermenia gen. nov. se propone para incluir a ésta y a Parahermenia piotrowskiae sp. nov. de Filipinas. Se proveen claves de identificación para las especies de Hermenia y Parahermenia.
Palabras clave: Caribe; Lepidonotus; Parahermenia; Escamosos; Taxonomía
Introduction
Grube (1850: 36) separated the species of Polynoe Savigny in Lamarck, 1818 after the cover of the dorsum by elytra; in one group he placed all species with elytra large, completely covering dorsum, and in the other, he placed all species with smaller elytra, leaving most of dorsal surface uncovered. In his following paper (Grube, 1851: 120), he added the number of cephalic appendages and separated one species (Polynoe muricata Savigny in Lamarck, 1818) because it has only 4 (no median antenna), and for the other species, he continued using the size of elytra or cover of dorsal surface. In these groups, he used the type of elytra, and their number, sometimes with additional details, to separate similar species, a method that is still used for the whole family.
Kinberg (1856) proposed several new families and genera of scaleworms, and described many species collected during the “Eugenie Expedition”. For the Polynoidae, he proposed 5 genera and diagnosed them, including the elytral cover of the dorsal surface. In Halosydna Kinberg, 1856 the dorsum could be exposed, and in Hermadion Kinberg, 1856, the posterior segments were uncovered.
Hermenia Grube & Örsted in Grube, 1856 was proposed after the finding of an unusual polynoid scale worm because it has a few large elytrae along anterior chaetigers, and minute elytrae in median and posterior segments. The body is robust, and not easily to be broken in parts, as is the case for other polynoids; further, the surface of the body instead of being smooth bears tubercles or papillae, its elytra are fleshy, firmly attached in their elytrophores, and elytra surface has spiny non-sclerotized tubercles and digitiform papillae.
Grube & Örsted in Grube (1856) proposed Hermenia for a species seemingly without palps. Treadwell (1911) noted the palps and corrected the diagnosis for the genus, redescribed H. verruculosa, and regarded Polynoe nodosa Treadwell, 1901 (non P. nodosa Sars, 1861) as a junior synonym. Seidler (1923) redescribed H. verruculosa and redefined the genus to include Lepidonotus acantholepis (Grube, 1876), and redescribed the latter species in a posterior publication (Seidler, 1924).
Hermenia includes H. verruculosa Grube & Örsted in Grube, 1856 from the Caribbean Sea, H. acantholepis (Grube, 1876) from the Philippines, and H. neoverruculosa Pettibone, 1975 from the Seychelles, Indian Ocean. The most recent revision in the genus was carried out by Pettibone (1975) who added the third species: H. neoverruculosa. Pettibone separated the 3 species after the presence of dorsal tubercles or papillae, the number of accessory teeth in neurochaetae, presence of ventral papillae, and number of anterior larger elytrae.
The Caribbean Sea species, H. verruculosa, has been recorded in many localities along the Western Atlantic: New Jersey, Bahamas, Gulf of Mexico, Antilles, and Central to South America, from intertidal down to depths of 220 m (Pettibone, 1975; Salazar-Vallejo, 1996). It has been observed in cracks and interstices of coral rocks (Treadwell, 1911), and it has been reported as living with the brittle starfish Ophiocoma pumilla (now Ophiocomella pumilla [Lütken, 1856] after O’Hara et al., 2019) (Devaney, 1974; Pettibone, 1993). On the other hand, the Indian Ocean species, H. neoverruculosa, is apparently restricted to a few localities, whereas H. acantholepis, described from the Philippines, has been recorded in many localities in the Western Pacific, in coral fragments or on sand (Hanley & Burke, 1991).
During the study of available specimens previously identified as H. verruculosa from different localities along the Grand Caribbean, some were found, indicating the presence of another species. The most relevant differences were the presence of a nuchal lappet (missing in H. verruculosa), the shape of anterior dorsal tubercles, and the pigmentation pattern. First, the anterior margin of chaetiger 2 can be projected anteriorly in a semicircular nuchal lappet, or not projected at all. Second, in most specimens the dorsal tubercles are globular, and in one species they are depressed, being wider than long if seen from above, whereas in a single species they are modified into thin papillae. Third, there are 2 pigmentation patterns; in H. verruculosa there is a rectangular white spot in chaetigers 5 and 6, and in some specimens, there can be paler areas in dorsal surface of parapodia of chaetiger 6, sometimes becoming an inverted T-shaped spot, in the other pattern, the dorsum along first 5-6 chaetigers is completely white, or has an inverted triangular white area. Other species have rather homogeneous pigmentation along the body; further, the dorsal cirrostyle of median segments can be whitish (H. verruculosa), or brownish. These differences were used to separate similar species.
Other morphological features were found to be too variable such as body shape (rectangular vs. fusiform), parapodial size is modified after body contraction, and notochaetae are thin, denticulate capillaries that are usually broken, sometimes from the base, such that their number or presence was regarded as irrelevant for separating similar species.
To standardize these characters, type material of all the described species was examined. Consequently, Hermenia is redefined, H. verruculosa is restricted, and 3 new species are described, 1 from the Grand Caribbean, another one from the Sunda Strait, Indonesia, and a third one from Vietnam. A key to identify all species in Hermenia is also included.
One species, Lepidonotus hermenioides Amoureux, 1974 described from Madagascar, resembles Hermenia species by having papillate integument and neurochaetae with 2-3 additional teeth, but differs from those species by having large elytra in median and posterior segments, each with fimbriate elytra. The finding of another similar species from the Philippines made us propose Parahermenia gen. nov. for including them, and the additional species is described as H. piotrowskiae sp. nov.
Materials and methods
This study was based on the analysis of type material of H. verruculosa, H. acantholepis, and H. neoverruculosa. Further, the study of many specimens previously identified as H. verruculosa from different localities in the Grand Caribbean were examined from the University of Miami, mostly made during their cruises now well-known as University of Miami Deep Sea Expeditions, on board of RVs Gerda and John Elliot Pillsbury (1964-1975), and specimens from the Mexican Caribbean deposited in the Colección de Referencia, ECOSUR, Chetumal (ECOSUR).
Specimens were examined with stereoscopes and with compound microscopes. The length of the specimens was measured from the prostomial anterior margin to the posterior pygidial border, body width was measured in the widest part of the body, including parapodia but excluding chaetae. Body, segmental and parapodial terms follow Pettibone (1975). Segments without elytrae but provided with dorsal cirri are called cirrigers, whereas those having elytrae are called elytrigers.
Drawings were carried out with a camera lucida; some specimens were photographed with a digital camera, and a series of focal successions were compressed with HeliconFocus Ver. 8. Plates were generated with Paint Shop Pro ver. 2021.
To standardize the differences between species the following features were used: a) nuchal lappet over the prostomium, which is an anterior projections from segment 2 (Fig. 5A); b) expansion and anterior projection of the first 2 elytrophores; c) width of the second segment dorsally and in its anterior part, being regarded as narrow if shorter than prostomial width (Fig. 2A), or wide if being as long as, or larger than prostomial width (Fig. 4B); d) parapodial size in comparison to body width, being regarded as long if longer than half body-width, or short if smaller than half body width; e) presence of notochaetae; f) abundance and prominence of dorsal tubercles; g) abundance of ventral papillae; h) size of median and posterior segments elytrae, being regarded as tiny if smaller than dorsal segmental width; i) elytral insertion, being regarded as embedded if not lying on the surface; j) body shape being cylindrical roughly rectangular, fusiform depressed wider anteriorly, swollen wider anteriorly.
Known species are presented in chronological sequence, whereas the newly described taxa will be arranged alphabetically. The material belongs to the following institutions and museums: BMNH: The Natural History Museum, London, England. CAS: California Academy of Sciences, Invertebrate Zoology, San Francisco, California, U.S.A. ECOSUR: Colección de Referencia, El Colegio de la Frontera Sur, Chetumal, Quintana Roo, México. LACM: Natural History Museum of Los Angeles County, Allan Hancock Polychaete collection, Los Angeles, California, U.S.A. MNHN: National Museum of Natural History, Paris, France. MZB: Museum Zoologicum Bogoriense, National Research and Innovation Agency, Cibinong, Indonesia. SMF: Senckenberg Museum, Frankfurt, Germany. UF: Florida Museum of Natural History, University of Florida, Gainesville, Florida, U.S.A. UMML: Museum of Marine Invertebrates, Rosenstiel School of Atmospheric and Marine Sciences, University of Miami, Florida, U.S.A. USNM: National Museum of Natural History, Smithsonian Institution, Maryland, U.S.A. ZMH: Zoologischen Museum und Institut, Hamburg (now Leibniz Institute for the Analysis of Biodiversity Change), Germany. ZMUC: Museum of Zoology, University of Copenhagen, Denmark.
Descriptions
Family Polynoidae Kinberg, 1856
Subfamily Lepidonotinae Willey, 1902
Hermenia Grube & Örsted in Grube, 1856
Hermenia Grube & Örsted in Grube, 1856: 44; Baird, 1865: 200 (diagnosis); Seidler, 1923: 261-262, Fig. 6; Seidler, 1924: 94, Pettibone, 1975. 234 (diagnosis, key); Wehe, 2006: 78 (diagnosis); Salazar-Vallejo & Eibye-Jacobsen, 2012: 1397.
Diagnosis (modificated of Pettibone, 1975). Lepidonotinae with short body; integument tuberculate or papillate, venter smooth or papillate. Body with 26 segments, 12 pairs of elytrae on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23. Elytra with globular macrotubercles and microtubercles, few papillae, fimbriae very short; first or first 3 elytra larger than median and posterior ones, most non-overlapping laterally or dorsally. Prostomium bilobed, with 2 pairs of eyes. Tentacular segment with few notochaetae and bulbous facial tubercle. Parapodia sub-biramous, notopodia small, neuropodia large. Notochaetae short, few to absent, slender, finely spinous. Neurochaetae stout, falcate, with 1-2 large accessory teeth. Dorsal cirri with cirrophores cylindrical, cirrostyles short. Ventral cirri short, subulate. Pharynx with 2 pairs of jaws and 11 pairs of marginal papillae. Nephridial papillae short, cylindrical from chaetigers 6-8.
Taxonomic summary
Type species. Hermenia verruculosa Grube & Örsted in Grube, 1856:44, by monotypy.
Distribution. Hermenia includes species from the Western Atlantic, the Indian and Western Pacific Oceans, that have been found mostly in shallow water mixed bottoms.
Remarks
Pettibone (1975) redefined Hermenia, but she did not regard the presence of the nuchal lappet as a distinctive character, although it is present in both H. acantholepis and H. neoverruculosa. The members of this genus are easily distinguished from other species belonging to lepidonotin genera provided with 12 pairs of elytra because, among other features, the reduction of the elytrae in medial and posterior segments, thick neurochaetae with separate accessory teeth, by having integument tuberculate or papillate with transverse lappet, and because the neurochaetae in segments 2 and 3 differ from those present in other segments (see key to genera below).
Hermenia resembles Parahermenia gen. nov. by having a tuberculate or papillate integument, neurochaetae subdistally smooth with tips bi- or tridentate. They differ because in Hermenia the first and last pair of elytra may overlap laterally, with most others being reduced, and by having minute fimbria with abundant macrotubercles; whereas in Parahermenia the elytra overlap laterally and have very long fimbria with few macrotubercles.
Key to Lepidonotinae with 12 pairs of elytra (modified after Fauchald, 1977)
1 With branchial filaments on the elytrophores
……………………………………………………………………………… 2
– Branchial filaments absent
……………………………………………………………………………… 4
2 (1) With pseudelytrae on non-elytrophoral, alternating segments posteriorly
……………………………………………………………………………… Euphionella Monro, 1936
– Pseudelytrae absent
……………………………………………………………………………… 3
3 (2) Neurochaetae coarsely spinose
……………………………………………………………………………… Chaetacanthus Seidler, 1924
– Neurochaetae finely plumose
……………………………………………………………………………… Euphione McIntosh, 1885
4 (1) Notopodia present
……………………………………………………………………………… 5
– Notopodia absent
……………………………………………………………………………… Drieschia Michaelsen, 1892
5 (4) All neurochaetae similar in thickness, without bidentate hooks
……………………………………………………………………………… 6
– With 1 very large bidentate hook in the middle of each neuropodial fascicle
……………………………………………………………………………… Sheila Monro, 1930
6 (5) Neurochaetae subdistally denticulate
……………………………………………………………………………… 7
– Neurochaetae subdistally with 1 or 2 accessory teth
……………………………………………………………………………… 9
7 (6) Notochaetae of 2 kinds, lancet-shaped and tapering and serrated
……………………………………………………………………………… Thormora Baird, 1865
– Notochaetae all of 1 kind, usually slender and serrated
……………………………………………………………………………… 8
8 (7) Pseudelytrae on cirrigerous segments (non-elytrophoral segments)
……………………………………………………………………………… Dilepidonotus Hartman, 1967
– Pseudelytrae absent
……………………………………………………………………………… Lepidonotus Leach, 1816
9 (6) All elytra overlapping laterally, with long fimbriae and a few macrotubercles
……………………………………………………………………………… Parahermenia gen. nov.
– A few anterior elytra overlapping laterally, most elytra small, non-overlapping laterally, fimbria very short, macrotubercles abundant
……………………………………………………………………………… Hermenia Grube & Örsted in Grube, 1856
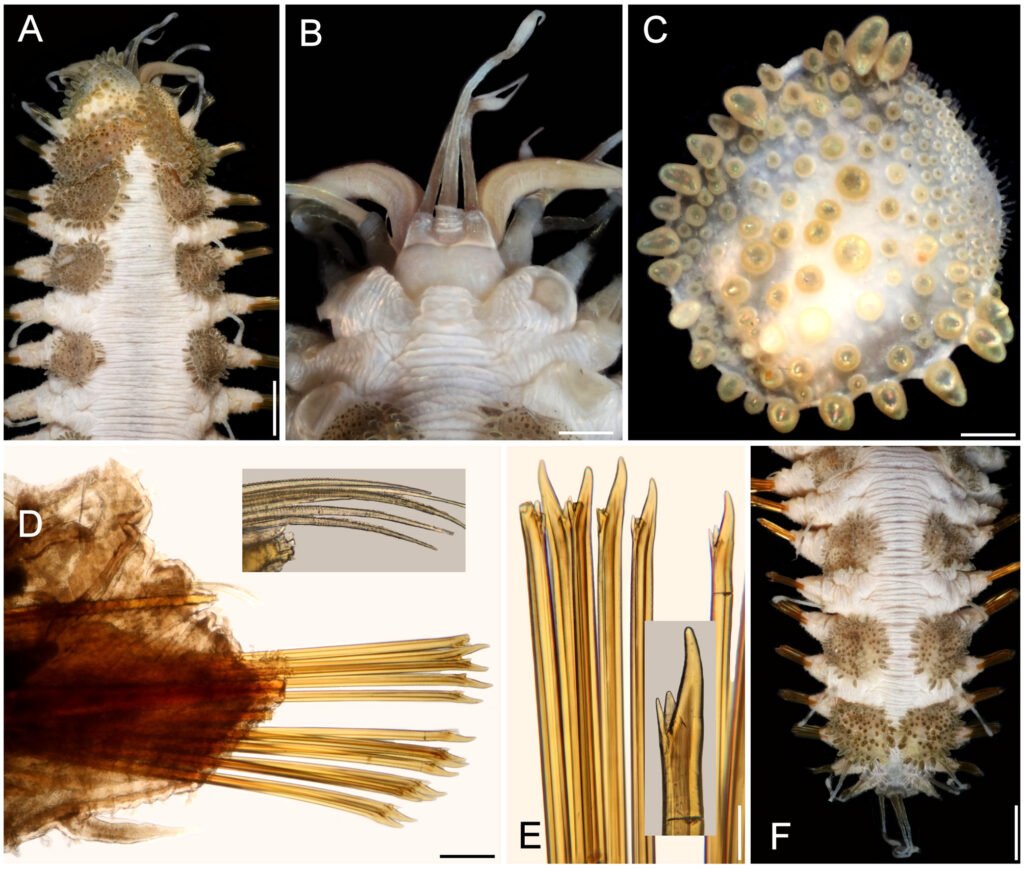
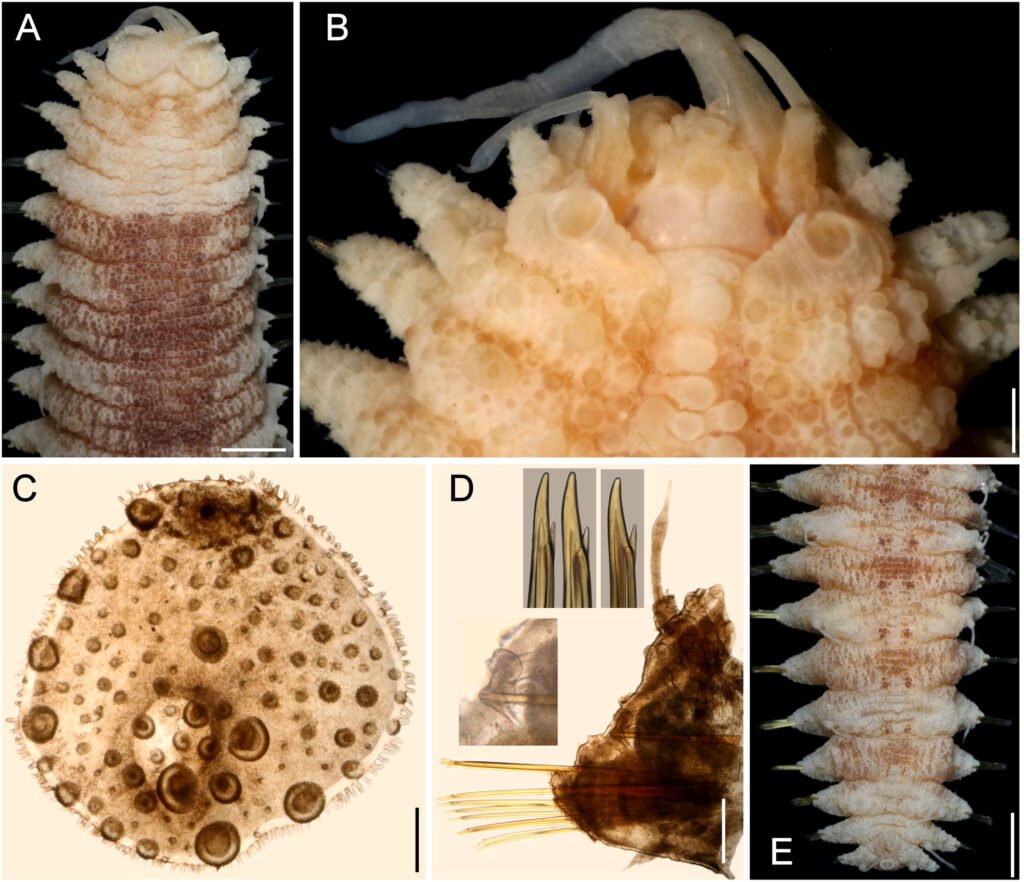
Hermenia verruculosa Grube & Örsted in Grube, 1856 restricted
(Figs. 1, 2)
Hermenia verruculosa Grube & Örsted in Grube, 1856: 44-45; Treadwell, 1911: 9-11, Figs. 23-26 (partim; Dry Tortugas, Florida, dead coral rock); Seidler, 1923: 261, Fig. 6 (Saint Thomas); Seidler, 1924: 95-96 (Antillas: Barbados and Saint Thomas); Hartman, 1939: 4 (Old providence Island, Colombia, reef in tide pool); Ebbs, 1966: 500, Fig. 4 (Margot Fish Shoal, Florida in coral debris and red coral patch); Pettibone, 1975: 235-238, Figs 1, 2 (synonyms, partim; Gulf of Mexico Florida, off Lousiana, Yucatán, Mexico, low water to 223 m).
Polynoe nodosa Treadwell, 1902: 187, Figs 8, 9 (non Sars, 1861; Fajardo, Puerto rico, Saint Thomas, 20-23 m, coral bottom).
Lepidonotus verruculosus: Horst, 1922: 198 (Caracas bay, Curazao in coral Porites).
Diagnosis. Hermenia with body subrectangular; first pair of elytra bigger, covering prostomium, remaining elytra tiny; dorsal integument with abundant globular tubercles, in segments 4 and 5 forming a pale; segment 2 not projected over prostomium as nuchal lappet; neurochaetae with 1 accessory tooth.
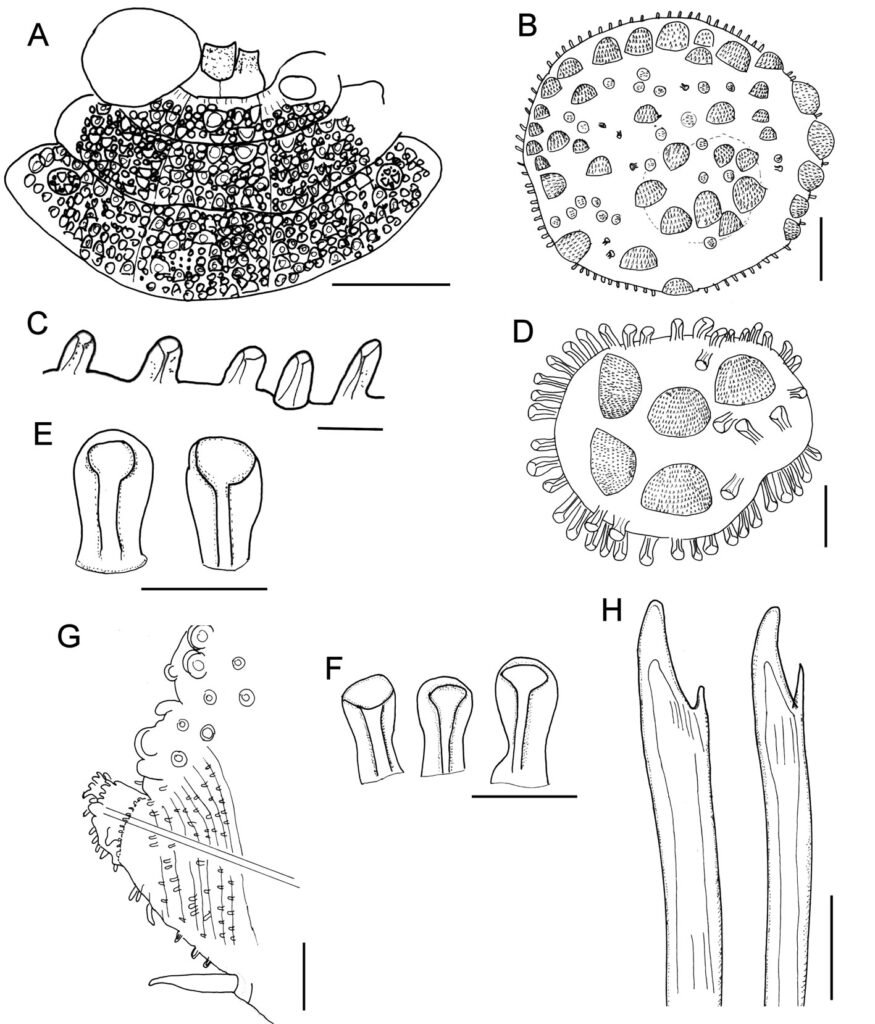
Description. Holotype (ZMUC-1101), complete, body dorsally curved, wider anteriorly, 25 mm long, 6.5 mm wide, 26 segments. Dorsum brownish with transverse pale and dark bands, each segment with dark or pale series of tubercles, densely packed, larger in anterior and posterior segments. Venter smooth or pilose, with abundant thin micropapillae. Segments 5 and 6 with globular pale or colorless papillae arranged in small diffuse pale spots, 1 per segment.
Prostomium bilobed (Fig. 1A), partially retracted into segment 2; facial tubercle round; eyes colorless, 2 pairs, dorsolateral, subdermal, posterior eyes faded color, hidden by peristomium. Median antenna with ceratophore prominent, annulate, inserted frontally, ceratostyle smooth, thin subdistally swollen, with a darker band, tip thin. Lateral antennae with ceratophores cylindrical, ceratostyles thick, smooth, half as long as median antenna, subdistally swollen, tips long. Palps lost.
Tentacular segment indistinct dorsally, without chaetae. Tentaculophores thick, cylindrical. Tentacular cirri unequal to each other, dorsal ones larger, as long as median antenna. Second segment, chaetiger, narrow mid-dorsally with a prominent globular tubercle (Fig. 2A, B), without nuchal lappet. Elytrophores not expanded, nor extended anteriorly (Fig. 1A).
First pair of elytra largest, colorless (Fig. 1B), completely cover prostomium; elytral surface with abundant macrotubercles and cylindrical papillae; macrotubercles wart-like, semispherical, spinulose, larger towards margin, concentrated posteriorly; papillae thick, shorter along elytral margin (Fig. 1C, D), slightly swollen distally (Fig. 1E); other elytra circular, of similar size, strongly adhered in the back, elytral surface with 4 large semispherical macrotubercles, spinulose, and scattered cylindrical papillae, elytral margins with abundant thick, digitate papillae (Fig. 1F).
Parapodia short, as long as half body width; dorsally with traverse arrays of globular tubercles along notopodium, remaining surface with arrays of papillae; ventrally with transverse pleats and scattered tiny papillae (Fig. 1G). Notopodia reduced, acicular lobe small, covered by a small papilla, without notochaetae. Neuropodia slantingly truncated, dorsally furrowed, prechaetal lobe longer than postchaetal one, distally with some papillae.
Cirrigerous segments with cylindrical, thick, short cirrophores, cirrostyles smooth, short, with some filiform papillae, not reaching neurochaetal tips. Ventral cirri with cirrophores thick, very short, cirrostyles thick, short, tapered. Nephridial papillae from segment 7, progressively larger posteriorly, becoming thick, long, cylindrical papillae.
Neurochaetae amber color. Segments 2 and 3 with neurochaetae thin and small; second segment with broken chaetae (after Pettibone [1975]) with long, entire tips and marginal large spines]. Segment 3 with 2 types of neurochaetae, upper ones with tips entire, marginally spinulose, lower ones bidentate, smooth. Following segments with thick neurochaetae, with a main tooth, thick, slightly falcate, tips sharp or blunt, and a single accessory tooth, thin, separated from the main one (Fig. 1H).
Posterior region tapered, truncate; pygidium with anus terminal, with 1 pair of translucent, long, subdistally swollen anal cirri.
Variation. The additional specimens have palps with abundant short papillae. In some specimens the pigmentation of the macrotubercles can be reddish, instead of dark brown, or light brown, but in most specimens, there is a small white spot including unpigmented tubercles middorsally between segments 4 and 5, sometimes it can be completely reduced such that the dorsum is brownish, or there can be 2 smaller spots over parapodia (Fig. 2A).
The nuchal lappet is reduced (Fig. 2B). First elytron can be almost triangular (Fig. 2C), probably after being modified by pharynx eversion. The pharynx is a cylindrical tube with 11 pairs of marginal papillae (Fig. 2D-E), with lateral papillae being smallest.
Parapodia biramous (Fig. 2F), but notochaetae often few, fragile, usually broken; neurochaetae usually brownish, with a darker core, and with a single additional subdistal denticle (Fig. 2F, inset). The posterior region is barely tapered (Fig. 2G), elytra are of similar size as those present in preceding segments; pygidium truncate with anus between chaetigers 25 and 26.
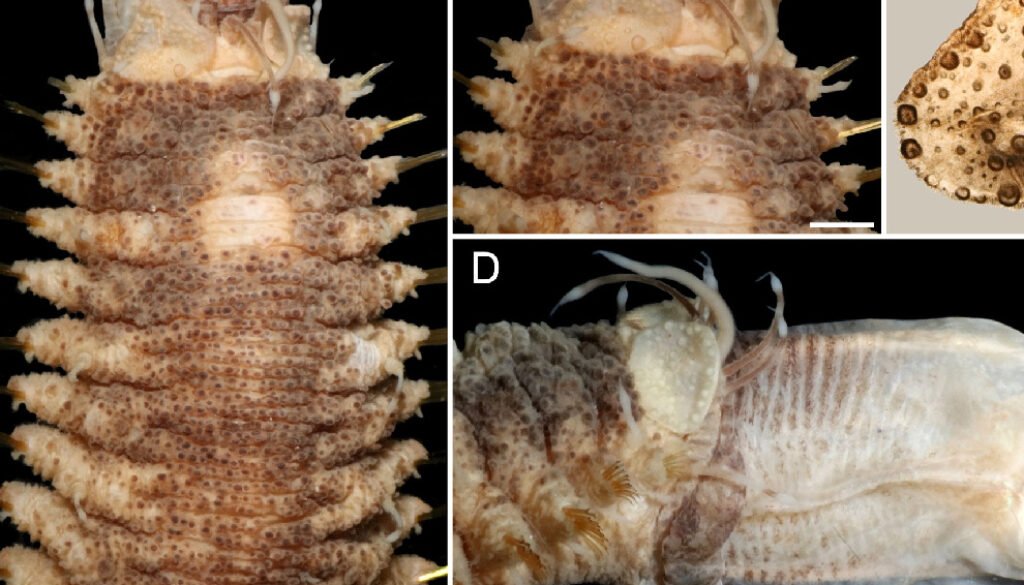
A small specimen (LACM 14774; 7.5 mm long, 3 mm wide) has morphological features present in larger specimens, such as the body shape, the pharynx is exposed and has 11 pairs of marginal papillae, with the midlateral ones smaller. The first elytra are markedly larger than the following ones, and it has many globular macro- and microtubercles, with surface echinulate, the fimbriae are slightly larger than 2 times longer than wide. Further, the parapodia are biramous and their integument is papillate, with few notochaetae, mostly 1 per ramus, and they are denticulate along a single side. The neurochaetae have a single accessory denticle, looking bifurcate, and lower neurochaetae tend to be sharper.
Taxonomic summary
Type material. Caribbean Sea, Virgin Islands. Holotype of Hermenia verruculosa Grube & Örsted, 1856 (ZMUC-1101), St. John, U.S. Virgin Islands, 6 Jun. 1846, coll. Krøyer. Smaller syntype of Polynoe nodosa Treadwell, 1902 (USNM 16013), Saint Thomas, U.S. Virgin Islands, USFCS Fish Hawk, Sta. 6079, 37-42 m, 6 Feb. 1899 (15 mm long, 3.5 mm wide); larger syntype (USNM 16014), Puerto Rico, Fajardo, US Steamer Fish Hawk, Sta. unumb., depth not given, probably intertidal, 17 Jan. 1899 (bent ventrally; first pair of elytra and left parapodia of chaetigers 2, 3 and 15-18, and right parapodium of chaetiger 20 previously removed (1 elytron, and 3 posterior ones left in container); paler dorsal transverse bands in chaetigers 8 and 10; venter with abundant tiny papillae. Body 25 mm long, 6 mm wide, 27 chaetigers).

Distribution. Widely recorded along the tropical Western Atlantic, in shallow water, mixed, rocky, or coral bottoms. The indication for the Eastern Pacific (Salazar-Vallejo & Eibye-Jacobsen, 2012) was wrong. The record for the Arabian Sea (Wehe 2006) is shown below to belong to a different, newly described species (see below).
Additional material. Florida. One specimen (UF 1878), Gulf coast, N of St. Petersburg (28.59° N, 84.26° W), rock bottom, sponges, 29 m, 13 Mar. 2011, G. Paulay, M. Bemis & J. Moore, coll. complete, slightly bent ventrally; posterior end removed for molecular studies; no nuchal lappet; white spot rectangular, with paler areas in chaetiger 6; dorsal cirrostyles with swollen area white; anus position unknown; body 12 mm long, 3.5 mm wide, 26 chaetigers).
One specimen (UF 1879), same data as above (complete, slightly bent ventrally; pharynx exposed; first pair of elytra, and right parapodium of chaetiger 14 removed for observation (kept in container); no nuchal lappet; white spot rectangular, with paler areas in chaetiger 6; dorsal cirrostyles with swollen area white; anus terminal in chaetiger 26; body 21 mm long, 7 mm wide, 26 chaetigers).
One specimen (UF 1885), same data as above (complete, slightly bent ventrally; right elytron 1, and right parapodium of chaetiger 12 removed for observation (kept in container); no nuchal lappet; white spot rectangular, with paler areas in chaetiger 6; dorsal cirrostyles with swollen area white; anus terminal in chaetiger 26; body 24 mm long, 6 mm wide, 26 chaetigers).
Two specimens (USNM 17733), Dry Tortugas, Jun. -Jul. 1914, A.L. Treadwell, coll. (markedly bent ventrally; larger one with pharynx fully exposed, with 10-11 marginal papillae; body 25-30 mm long, 4.5-6.0 mm wide, 26 chaetigers).
Two specimens (USNM 46913), Tortugas,1930, W.L Schmitt, coll. (only 1 specimen in container; bent ventrally; chaetiger 6 with 3 white spots dorsally; 2 parapodia and first elytron from another specimen in container); body 30 mm long, 8 mm wide, 26 chaetigers).
Caribbean Sea. One specimen (ECOSUR 1309), University of Miami, Cruise P6806, RV Pillsbury, Southern Caribbean, off NE Venezuela, Sta. 709 (11°08.8’ N, 62°46.1’ W), 46 m, 19 Jul. 1968 (complete, anterior region almost completely brownish, with 1 middorsal small white spot in chaetiger 5, 2 laterals in chaetiger 6; no nuchal lappet; anus terminal in chaetiger 26; body 28 mm long, 6 mm wide, 26 chaetigers).
One specimen (ECOSUR 1310), University of Miami, Cruise P6802, RV Pillsbury, Nortwestern Caribbean, off Honduras, Sta. 629 (15°58’ N, 86°09’ W), 40 m, 21 Mar. 1968 (complete, anterior region with 1 middorsal small white spot in chaetiger 5, 2 laterals in chaetiger 6; no nuchal lappet; anus terminal in chaetiger 26; body 26 mm long, 7 mm wide, 26 chaetigers).
One specimen (ECOSUR 1311), Majagual, reef lagoon, rocks, 4 m, 6 Jun. 1998, M.A. Ruiz-Zárate, coll. (bent ventrally; with small narrow middorsal white and 2 lateral white spots in chaetiger 6; no nuchal lappet; anus terminal in chaetiger 26; body 18 mm long, 5 mm wide, 26 chaetigers).
Three specimens (ECOSUR 1316), Majagual, reef lagoon, rocks, 4 m, 6 Jun. 1998, M.A. Ruiz-Zárate, coll. (juveniles, bent ventrally, 1 with pharynx exposed, 10 pairs of marginal papillae; with wide middorsal white spot in chaetigers 4-5, and 2 lateral white spots in chaetiger 6; no nuchal lappet; anus terminal in chaetiger 26; body 8.1-8.5 mm long, 2.0-2.1 mm wide, 26 chaetigers).
One specimen (ECOSUR 3289), Majagual, Quintana Roo, México, reef lagoon, 4 m 6 Jun. 1998, M.A. Ruiz-Zárate, coll. (bent ventrally; pharynx exposed, 11 pairs of marginal papillae; 3 round white spots in chaetiger 6, anus terminal in chaetiger 26; body 13 mm long, 4 mm wide, 26 chaetigers).
One specimen (ECOSUR 3289), Punta Nizuc, Quintana Roo, México, 4 m, 10 Feb. 2001, P. Salazar-Silva, coll. (bent ventrally; with white middorsal band in chaetiger 5, wide transverse band in chaetiger 6; no nuchal lappet; anus terminal in chaetiger 26; body 11 mm long, 3 mm wide, 26 chaetigers).
One specimen (LACM 14763), British Virgin Islands, Guana Island, Muskmelon Bay, off Crab Cove (18.48° N, 64.57° W), reef slope coral heads, 10-16 m, 13 Jul. 2000, G. Hendler, T. Zimmerman, J. Martin & R. Ware, coll. (bent ventrally, with salt particles adsorbed on body; chaetigers 5-6 with a median rectangular white spot, and 2 smaller lateral ones on chaetiger 6; body 20 mm long, 6 mm wide, 26 chaetigers).
Two specimens (LACM 14774), British Virgin Islands, Guana Island, Pelican Ghut (18°28’36” N, 64°33’31” W), 6-12 m, G. Hendler, coll. (body 7.5-18.0 mm long, 3.0-6.5 mm wide, 26 chaetigers; smaller specimen features included in variation).
One specimen (LACM A44-39), Tortuga Island, RV Velero III, Sta. 44-39 (11°02’30” N, 65°14’45” W to 11°03’30” N, 65°14’00” W), 38-40 m, 21 Apr. 1939 (bent ventrally; chaetiger 6 with a median and 2 lateral white spots; body 23 mm long, 6 mm wide, 26 chaetigers).
Two specimens (LACM 147856), British White Bay, ARMS (18.3° N, 64.37° W), 9 m, 15 Jul. 2000, J. Zimmerman, J. Martin & R. Ware, coll. (almost colorless; both without nuchal hood, pharynx exposed, with 10 pairs of marginal papillae, body 6-8 mm long, 1.5-2.0 mm wide, 25-26 chaetigers).
One specimen (UMML 22.729a), University of Miami, Cruise P7006, Hispanola and Jamaica, RV Pillsbury, Sta. 1198 (17°49.4’ N, 76°12.3’ W), 29-37 m, 4 Jul. 1970 (bent ventrally; with 3 white spots, 1 in chaetigers 5-6, 2 laterals in chaetiger 6; without nuchal lappet; anus terminal in chaetiger 26; body 20 mm long, 6 mm wide, 26 chaetigers).
One specimen (UMML P979), University of Miami, Cruise P6907, Antillean Arc, Leeward Islands, RV Pillsbury, Sta. 979 (17°51’ N, 62°39’ W), 37 m, 22 Jul. 1969 (anterior fragment; without nuchal lappet; colorless, 10 mm long, 5 mm wide, 16 chaetigers).
One specimen (USNM 46916) Ascención Bay, Quintana Roo, Mexico, Central Part of Nicchehabin Reef., 1.2-1.8 m, Apr. 1960, E.L. Bousfield, coll. (slightly twisted; brownish, white spot in chaetiger 6; posterior nephridial lobes brownish; body 19.5 mm long, 5 mm wide, 26 chaetigers).
One specimen (USNM 46917), Ascensión Bay, Nicchehabin Reef., Quintana Roo, México, 21-19 m, 13 Apr. 1960, E.L. Bousfield, coll. (bent ventrally; dorsum with 3 white spots in chaetiger 6, chaetiger 5 with a median spot; body 28 mm long, 6 mm wide, 26 chaetigers).
One specimen (USNM 46920) Majito Reef, Puerto Rico, 0.3-0.6 m, 9 Mar. 1967, M.E. Rice, coll. (bent laterally; median segments swollen; body 20 mm long, 6 mm wide, 26 chaetigers).
Two specimens (USNM 50112), Carrie Bow Cay, Belize, 30 m, 11 Jun. 1972, M.E. Rice, coll. (bent ventrally; pharynx fully everted, 11-12 marginal papillae; body 16-21 mm long, 4-7 mm wide, 25-26 chaetigers).
One specimen (ZMUC-1102), St. Croix Island, H. Riise, coll. (no further data; prostomium retracted into second segment, without palps; body 19 mm long, 6.5 mm wide, 25 chaetigers).
Remarks
Hermenia verruculosa Grube & Örsted in Grube, 1856 differs from other species in the genus (see Hermenia key below)because it has a subrectangular dorsal white spot; segment 2 wide among the elytrophores, not projected on the prostomium as a nuchal lappet, elytrophores not expanded, dorsum with prominent globular densely packed tubercles; between segments 4 and 5 there is a dorsal small spot diffuse to well defined of unpigmented tubercles; venter wrinkled with abundant short thin papillae; elytra fleshy, rounded, attached firmly. The first pair of elytra is larger than the middle and posterior ones. All elytra with macrotubercles semispherical, spinous, unpigmented on the first pair of elytra, on subsequent elytrae reddish. Parapodia short, neuropodia diagonally truncate with an incipient prechaetal lobe. Neurochaetae with 1 accessory tooth and notochaetae absent in most of the parapodia. Pharynx everted with 11 pairs of marginal papillae and 2 pairs of jaws. The dorsal spot of unpigmented tubercles (Fig. 2F) is herein considered as distinctive of H. verruculosa.
Hermenia verruculosa has been recorded from different localities in the Grand Caribbean (Fauvel, 1953b; Horst, 1922; Bellan, 1964); however, the characterizations were short, often without illustrations, and the specimens are not available. Ebbs (1966) recorded 2 specimens from a coral patch of Margot Fish Shoal, Miami, Florida, as H. verruculosa, and his description referred red pigmentation; these specimens are colorless now, and the tubercles are white. On the other hand, part of Ebbs’ material corresponds with the features of H. verruculosa (UMML 150), whereas another lot (UMML 22:149) includes what we regard as an undescribed species described below.
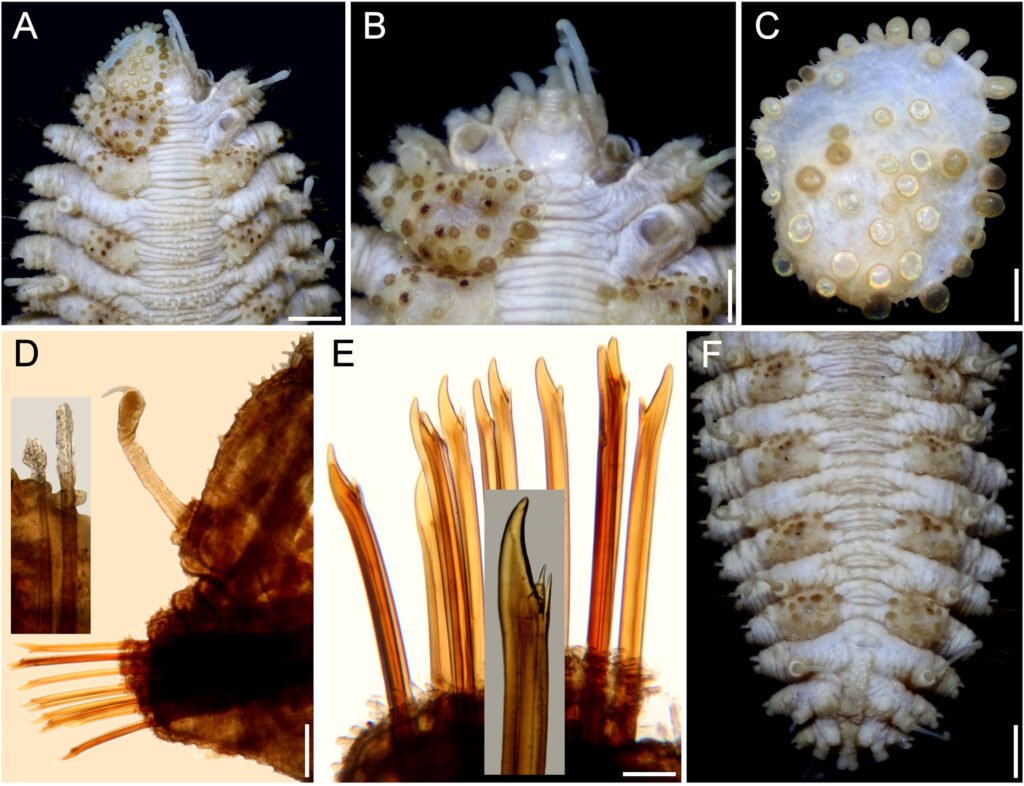
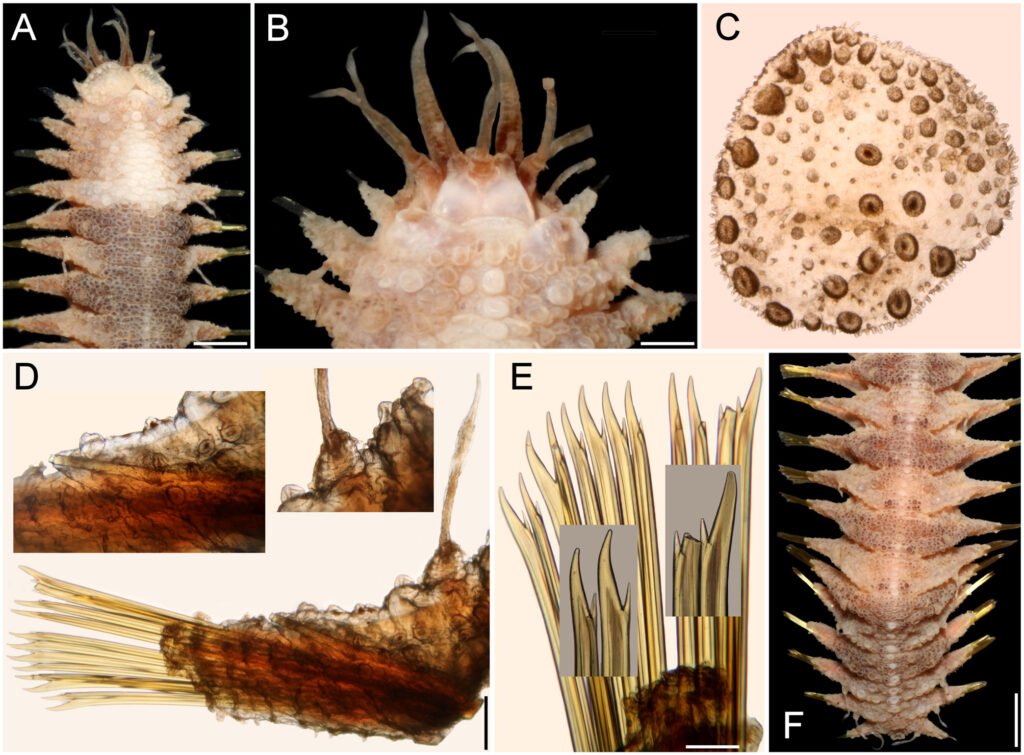
Hermenia acantholepis (Grube, 1876) restricted
(Figs. 3, 4)
Polynoe acantholepis Grube, 1876: 61.
Polynoe (Lepidonotus) acantholepis: Grube, 1878: 24, Pl. 2, Fig. 1 (Samoa y Philippines).
Lepidonotus acantholepis: Herdman & Hornell, 1903: 25 (Ceylan, currently Sri Lanka, 82-92 m, in coral); Fauvel, 1922: 490-491, Fig. 1 (Houtman Abrolhos, Indian Ocean, in coral reef).
Hermenia acantholepis: Seidler, 1923: 262; Seidler, 1924: 94-95 (North West of Madagascar); Monro, 1924: 39-40, Figs 2-3 (Goto Island, Japon); Pruvot, 1930: 11-13, Pl. 1, Figs 27-32 (syn.); Fauvel, 1932a: 16-17 (Indian Ocean); Fauvel, 1947: 16-17, Fig. 12 (syn.), New Caledonia, South Pacific; Fauvel, 1953a: 38, Fig. 14a, b (Ceylan currently Sri Lanka); Pettibone, 1975: 239-241, Fig. 3 (partim, Fig. 4 corresponds to H. mezianei sp. nov., see below); Hanley & Burke, 1991: 62-64, Fig. 19A-G (Chesterfield Islands, New Caledonia, coral shallow water to 69 m, coral sand).
Diagnosis. Hermenia with body subrectangular; first and last pairs of elytra larger, overlapping, first 3 pairs larger than following ones; median elytra circular covering adjacent segments; elytral tubercles globular, with a basal hump; dorsal integument white, almost smooth, with thin papillae; segment 2 briefly projected over prostomium as a small nuchal lappet; neurochaetae tridentate.
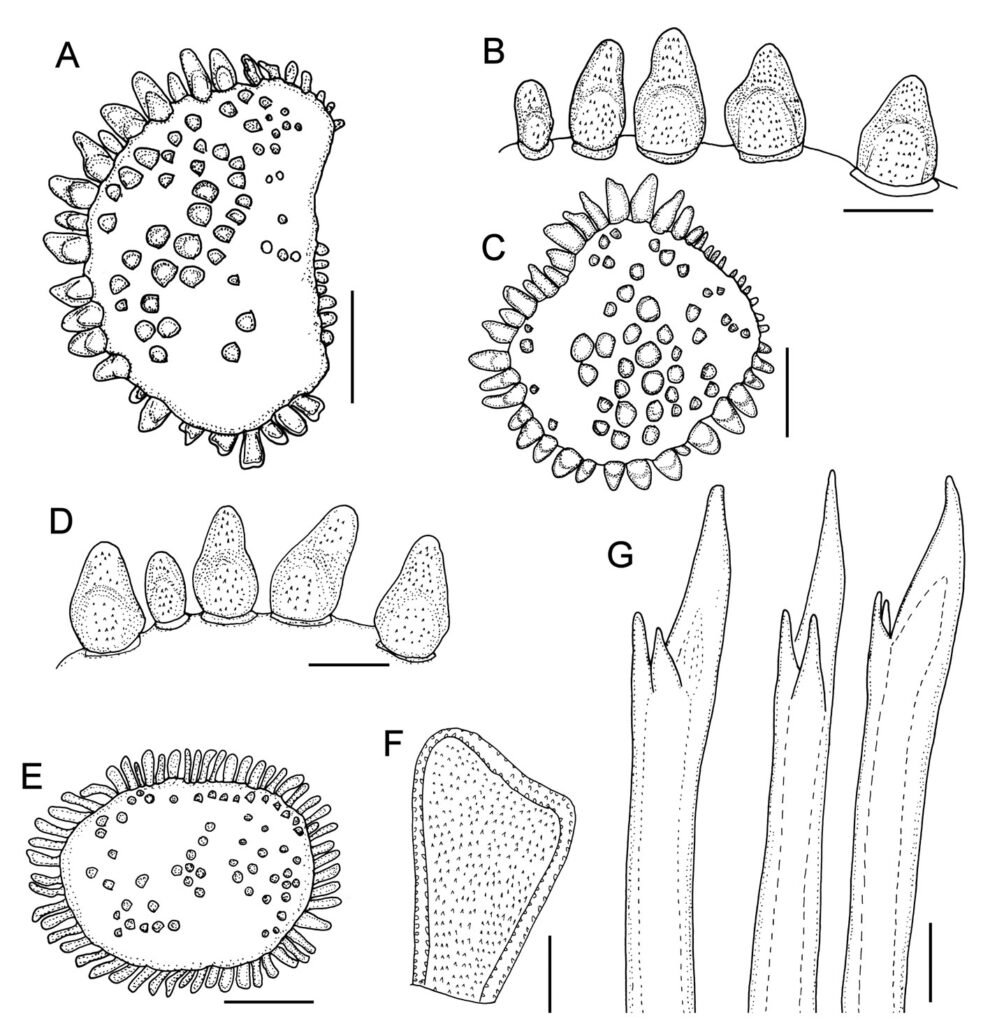
Description. Holotype (ZMH 504) complete, mature female; dorsum wrinkled, globular tubercles scarce, whitish; venter wrinkled, without papillae; body depressed, 24 mm long, 6.5 mm wide, 26 chaetigers.
Prostomium in bad condition, wider than long, partially covered by segment 2; facial tubercle round; 2 pairs of eyes, both almost unpigmented. Median antenna with ceratophore cylindrical, long, inserted frontally; ceratostyle long, thick, surface smooth, without papillae; subdistally swollen, tip filiform. Lateral antennae with ceratophores thin, ceratostyle shorter, similar in shape to median antenna. Palps thick, without pigmentation, surface with rows of short papillae.
Tentacular segment not visible dorsally, tentaculophores thick, without chaetae; tentacular cirri similar in shape to median antennae. Segment 2 with nuchal lappet. Elytrophores expanded, not covering tentaculophores.
Elytra small, not overlapped laterally nor covering dorsum; elytra of anterior and posterior segments larger than elytra of middle segments, first pair of elytra round (Fig. 3C), second pair reniform (Fig. 3A); median and posterior elytra round (Fig. 3E), covering at least half width of adjacent segments. Elytral surface with tubercles and abundant filiform papillae. Tubercles abundant, ovoid, spinous, basally bulbous, projected beyond elytral margin (Fig. 3B, D). Elytra of median and posterior segments with marginal macrotubercles thinner, compressed (Fig. 3E-F), mostly brownish.
Parapodia with notopodia reduced, cylindrical ridge over neuropodia. Neuropodia thick, surface with transverse wrinkled rows, prechaetal lobe truncate, slightly larger than postchaetal lobe, distally with short papillae.
Dorsal cirri short, not longer than neuropodia, expanded subdistally, tips short filiform, similar in shape to antennae. Cirrophore cylindrical, short. Ventral cirri short, basally thick, tapered into fine tips, surface smooth. Nephridial papillae thick, short, distally blunt along chaetigers 6-25.
Notochaetae of anterior and middle segments short, scarce, upper region long, tapered into fine tip, absent in posterior segments. Neurochaetae thick, with striae; upper region short; tips thick, slightly curved, with 2 short, subdistal accessory teeth (Fig. 3G).
Posterior region tapered, blunt; pygidium with anus dorsal between chaetigers 25 and 26, anal cirri missing (thin, distally swollen, as long as last segment).
Variation. One specimen recently collected from Indonesia (UF 41) has a body almost pure white with brownish elytra (Fig. 4A); first 3 pairs of elytra larger than median ones, progressively smaller, such that the third is about twice as large as the fourth. The prostomium is white, with darker ceratophores and a basal medial region of ceratostyles, with a small dark band before swollen areas (Fig. 4B); eyes are black, of similar size, anterior eyes in the widest prostomial area, posterior ones towards the posterior margin. The nuchal lappet is truncate, well defined (Fig. 4B). The elytra have brownish macrotubercles, larger ones are marginal, with a basal swollen area, and globular microtubercles, more abundant along the inner anterior region (Fig. 4C). Parapodia biramous with small notochaetae (Fig. 4D, inset). Neurochaetae brownish with darker core, each with 2 subdistal teeth, often of similar size but different width (Fig. 4E). The posterior region is slightly tapered, with last pair of elytra slightly larger than preceding ones (Fig. 4F); anus dorsal, in posterior margin of chaetiger 25, anal cirri thin, resembling dorsal cirri, as long as last segment.
Taxonomic summary
Type material. Holotype of Polynoe acantholepis Grube, 1876 (ZMH 504), Upolu, Philippines.
Distribution. Indian Ocean and Western Pacific (Horst, 1917; Monro, 1939; Hanley & Burke, 1991; Imajima, 1997), in shallow mixed bottoms.
Additional material. Sri Lanka. One specimen (BMNH 1973:12), Talili, 73 m, no further data (dorsum wrinkled, with few tiny tubercles; venter with small papillae; first and second pair of elytra covering prostomium; elytral macrotubercles projected beyond margin, other elytra with marginal papillae filiform; notopodia small, with an acicular papilla).
Maldives. One specimen (BMNH 1941.4.4.195), Felidu, J.S. Gardiner, coll. (dorsum pale color, wrinkled with few papillae; notopodia short lobes; dorsal papillae short, compressed; venter with abundant short papillae; elytra of middle segments smaller than those of anterior segments, but larger than posterior ones).
Andaman Sea. Two specimens (LACM 2831963), juveniles, International Indian Ocean Expedition, RV Anton Bruun, Sta. 29 (11°23’ N, 93°31’ E), 55-40 m, 28 Mar. 1963 (1 dried-out; complete, whitish, cephalic appendages lost; elytra mostly white, barely compressed; nuchal lappet indistinct after eversion of pharynx; 11 pairs of marginal papillae; left parapodium of chaetiger 12 removed for observation kept in container; body 8 mm long, 2.5 mm wide, 26 chaetigers).
Indonesia. One specimen (UF 41), Sulawesi (Celebes Island), southern outer barrier reef (0.49° S, 122.07° E), 1-3 m, 22 Sep. 1999, G. Paulay, coll. (bent ventrally; body white, corrugated, pilose; elytra brownish, marginal macrotubercles L-shaped, internal lobe shorter; first elytra and left parapodium of chaetiger 14 removed for observation, kept in container; nuchal lappet distinct; dorsal cirrostyles with swollen area white; anus in chaetiger 25; body 23 mm long, 6 mm wide, 26 chaetigers).
Philippines. One specimen (CAS 187233), Hearst Philippine Biodiversity Expedition 2011, Luzon, Batangas Province, Mabini (Calumpan Peninsula), Maricaban Strait, Arthur’s Rock (13.70° N, 120.87° E), 16 m, 10 May 2011, A. Hermosillo, coll. (bent ventrally; body dirty white, first elytra whitish, all others brownish; first 3 and last elytra with marginal macrotubercles compressed, basally expanded (L-shaped); nuchal lappet short; dorsal cirrostyles with swollen areas white; anus in chaetiger 25; body 43 mm long, 9 mm wide, 26 chaetigers).
One specimen (CAS 214669), Verde Island Passage Expedition 2015, Mindoro, Oriental Mindoro Province, Puerto Galera, Shipyard Dive Site (13.52° N, 120.96° E), 6.5-16.0 m, sandy mud, 12 Apr. 2015, C. Piotrowski, coll. (partially dehydrated; middorsal area grayish, laterally white, elytra elytra brownish; first 3 and last elytra with marginal macrotubercles compressed, basally expanded (L-shaped); nuchal lappet distinct; dorsal cirrostyles with swollen areas white; anus between chaetigers 25-26; body 18.5 mm long, 5 mm wide, 26 chaetigers).
Papua New Guinea. One specimen (MNHN IA 2015-1924), Kavieng 2014 Expedition, New Ireland, RV Alis, Sta. DW4492 (02°25’23.99’’ S, 149°57’35.42’’ E), 112-140 m, 6 Sep. 2014 (bent ventrally, dried-out; first left elytron without macrotubercles along anterior inner area; median and posterior elytra circular, last pair larger than previous one; body 37 mm long, 7 mm wide, 26 chaetigers).
Coral Sea. One specimen (MNHN IA 2023-19), Cruise CORAIL 2, RV Coriolis, Sta. DW31 (19°24’51.59’’ S, 158°45’1.81’’ E), 57 m, 23 Jul. 1988, B. Richer de Forges, coll. (complete, slightly bent ventrally, first left elytron without macrotubercles along anterior inner area; median and posterior elytra circular, last 2 pairs larger than previous ones; body 22 mm long, 5.5 mm wide, 26 chaetigers).
Japan. One specimen (BMNH 1925.1.28.4-5), Goto Island, no further data (dorsum wrinkled without tubercles or papillae; dorsum smooth; notopodia cylindrical ridges; coelom with numerous oocytes).
New Caledonia. One specimen (MNHN IA 2023-57), Musorstom Cruise LAGON, Yaté Sector, RV Vauban, Sta. DW642 (21°54’12.0024’’ S, 166°42’12.0204’’ E), 44-47 m, 7 Aug. 1986, B. Richer de Forges, coll. (complete, slightly bent ventrally, first left elytron without macrotubercles along anterior inner area; median and posterior elytra circular, last pair larger than previous ones; body 21 mm long, 6 mm wide, 26 chaetigers).
One specimen (MNHN IA 2023-58), Musorstom Cruise LAGON, Yaté Sector, RV Vauban, Sta. DW737 (22°08’23.99’’ S, 166°59’06’’ E), 49-50 m, 12 Aug. 1986, B. Richer de Forges, coll. (complete, slightly bent ventrally, first left elytron without macrotubercles along anterior inner area; median and posterior elytra circular, last pair larger than previous ones; body 17.5 mm long, 5.5 mm wide, 26 chaetigers).
Remarks
Hermenia acantholepis differs from H. verruculosa by having a depressed body; segment 2 wide projected on the prostomium as a short nuchal lappet; dorsum with transverse wrinkled ridges, tubercles small, flattened, scarce. Venter smooth, without papillae. Elytra with 2 kinds of tubercles, bulbous-ovoid basally and larger around margin. In earlier species diagnoses the marginal macrotubercles have been regarded as ovoid, but they are roughly bottle-shaped, with a basal swollen area; H. acantholepis is also distinguished by its neurochaetae because they have 2 subdistal accessory teeth, and notochaetae are present along most notopodia.
Pruvot (1930: 11) and Fauvel (1947: 17) noted that H. acantholepis has a milky white body, with brownish elytra, and dorsal cirri with brownish bases. Pruvot (1930: 13) also noted 2 varieties: the western one found from Sri-Lanka to the Philippines with very small elytra (matching H. neoverruculosa Pettibone, 1975, see below), and an eastern one, found in New Caledonia and Samoa, with larger elytra (matching the current H. acantholepis).
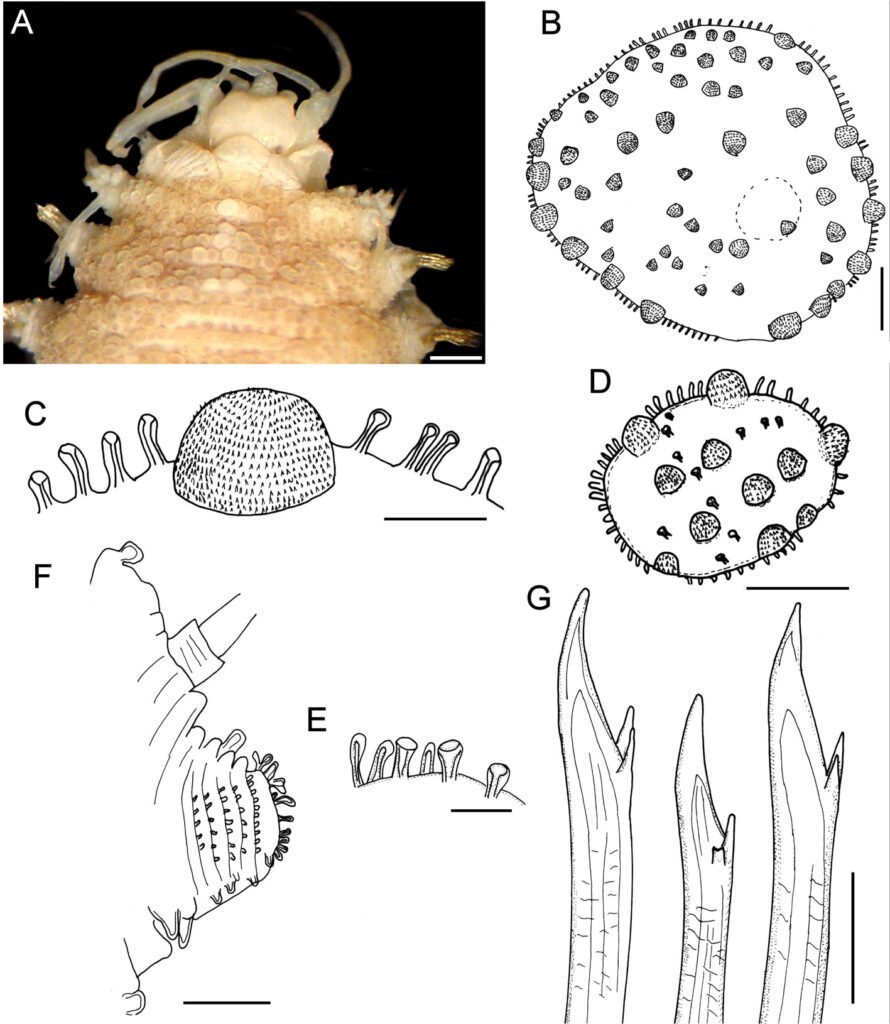
Hermenia neoverruculosa Pettibone, 1975
(Figs. 5, 6)
Hermenia acantholepis: Fauvel, 1932b: 132 (Gulf of Suez, Read Sea); Fauvel, 1933: 41 (Gulf of Suez, Read Sea); Wehe, 2006: 79, Fig. 13a-c. (non Grube, 1876; Red Sea).
Hermenia neoverruculosa Pettibone, 1975:242-245, Figs 5-6 (syn.).
Diagnosis. Hermenia with body subrectangular; first and second pair of elytra overlapping; dorsal integument with abundant globular tubercles, often with black core; segment 2 with nuchal lappet wide, round; median and posterior elytra with black macrotubercles; neurochaetae tridentate.
Description. Holotype (BMNH 1941.4.4.197) complete, body depressed, subrectangular, 30 mm long, 6.5 cm wide, 26 chaetigers. Dorsum with transverse rows of globular tubercles with darker cores, larger along anterior and posterior segments. Tubercles light brown, except on segments 3-4, where some tubercles are whitish, without pigmentation, forming a small, diffuse middorsal spot. Venter pale, wrinkled, pubescent.
Prostomium bilobed (Fig. 5A), wider than long, not retracted into segment 2; facial tubercle round, pale. Eyes black; anterior eyes directed anteriorly, on the widest part of the prostomium, subdermal; posterior eyes near posterior margin, darker, slightly larger Fig. 6B). Median antenna with ceratophore inserted frontally, thick, surface with papillae, ceratostyle tubular, 2 times as long as prostomium length, subdistally swollen, tip filiform, surface smooth, with dark bands; lateral antennae with ceratophores cylindrical, short, about as long as prostomium; ceratostyles shorter, resembling median antenna. Palps missing.
Tentacular segment not visible dorsally, tentaculophores thin, long, with scarce, short chaetae. Tentacular cirri thin, long, resembling antennae, surface smooth.
Segment 2 narrow with a wide rounded nuchal lappet. First pair of elytrophores reaching bases of tentaculophores. Nephridial papillae not visible along anterior segments, distinct from chaetiger 7, longer along median and posterior segments.
First 2 pairs of elytra larger than following ones; elytron 3 slightly larger than elytron 4; first pair of elytra overlapped dorsally; elytral surface with macrotubercles blackish (Fig. 5B), or pale, and papillae; macrotubercles scattered, ovoid, short to elongate, blunt, spinous, micropapillae cylindrical, abundant (Fig. 5C), thicker along margin. Elytra of median and posterior segments small (Fig. 5D), macrotubercles black, with abundant cylindrical papillae on surface and along margins (Fig. 5E).
Parapodia with notopodia reduced to acicular lobe, acicular papilla present (Fig. 5F). Neuropodia thick, robust, distally with small papillae, and small prechaetal and postchaetal lobes with long papillae, postchaetal lobe larger than prechaetal one; dorsal surface with transverse rows of globular pale tubercles. Dorsal cirri thin, smooth, short, not reaching neurochaetae tips, same shape as antennae; cirrophore short, cylindrical. Ventral cirri short, thin, not reaching the lower tip of the neurochaetal lobe.
Notochaetae thin, short, 2-3, laterally denticulate capillaries. Neurochaetae thick, upper region short, main fang long, thick, slightly curved, more curved in posterior segments, accessory teeth short, 1/4 as long as main fang. Second parapodia with thin neurochaetae, tips entire with 3 long spines. Median parapodia with neurochaetae often with 2 accessory teeth, sometimes 1 broken (Fig. 5G).
Posterior region tapered, truncate; pygidium with anus dorsal, anal cirri lost.
Variation. One specimen (UF 3985) has body grayish, dorsal tubercles with cores slightly darker, better defined in intersegmental areas; first elytra pale with macrotubercles blackish, other elytra grayish with dark macrotubercles; first and second pair of elytra of similar size, second pair about 3 times as large as third pair (Fig. 6A); following elytra small, round, with black macrotubercles.
Prostomium pale, with ceratophores blackish with pigmentation extended along anterior prostomial areas, ceratostyles brownish, with a wide brownish band before enlarged pale area, tips pale (Fig. 6B); nuchal lappet barely developed, truncate. First elytron (Fig. 6C) with marginal macrotubercles lobate, not basally expanded, inner macrotubercles globular, shorter, blackish and paler ones (Fig. 6C).
Parapodia biramous (Fig. 6D) with few notochaetae (Fig. 6D, inset); neurochaetae abundant, brownish and paler, with darker cores (Fig. 6E), each with 2 fragile, tapered accessory teeth, sometimes with a single denticle left (Fig. 6E, inset), or all broken. Posterior region tapered; last pair of elytra as large as preceding ones, with black macrotubercles; anus dorsal between chaetigers 25-26, anal cirri thin, delicate, as long as last chaetiger (Fig. 6F).
Taxonomic summary
Type material. Republic of Mauritius. Holotype of Hermenia neoverruculosa Pettibone, 1975 (BMNH 1941.4.4.197), Cargados Carajos, 55 m, Sea Lark Expedition, 28 Aug. 1905, J.S. Gardiner, coll.
Distribution. Indian Ocean to Western Pacific, in shallow water mixed bottoms.
Additional material. Red Sea. One specimen (MNHN A403), Mission Dollfus en Égypte 1927-1929, Sta. 24, ‘bloc des vermets’ (probably intertidal Dendropoma vermetid mass, see Ben-Eliahu 1975), 30 Dec. 1928, R.P. Dollfus, coll. (complete, bent ventrally, partially dehydrated, integument damaged, middorsally eroded, with fine sediment particles laterally; dorsum with tubercles; all elytra on site, with fine sediment particles, median and posterior elytra circular; anus between chaetigers 25-26; body 14.5 mm long, 4 mm wide, 26 chaetigers).
One specimen (MNHN A894), Mission Dollfus en Égypte 1927-1929, Sta. 24, ‘bloc des vermets’ (probably intertidal Dendropoma vermetid mass, see Ben-Eliahu 1975), 30 Dec. 1928, R.P. Dollfus, coll. (complete, breaking in 2 parts, integument damaged, almost completely detached from body wall; dorsum with tubercles; 2 anterior elytra and right parapodium of chaetiger 12 previously removed, kept in container; most elytra on site, median and posterior ones circular; anus between chaetigers 25-26; body 18.5 mm, long, 5 mm wide, 26 chaetigers).
Papua New Guinea. One specimen (MNHN IA 2017-3596), Papua Niugini Expedition, W. Wongat Island, Sta. PR108 (05°08’04.44” S, 145°49’29.21” E), 2-30 m, 26 Nov. 2012 (complete, bent ventrally; all elytra on site, median elytra circular, smaller than segment width; anterior end markedly retracted, nuchal lappet not seen; dorsal tubercles globular, with black core; venter pubescent; anus dorsal between chaetigers 25-26; body 45 mm long, 8 mm wide, 26 chaetigers).
One specimen (UF 3985), Madang Province, BilBil Island (5.2962° S, 145.7822° E), outer reef, 13 m, 12 Nov. 2012, B. Faure, R. Ibik & P.-H. Kuo, coll. (complete, slightly bent laterally; first pair of elytra and right parapodia of chaetigers 10 and 12 removed for observation, kept in container; nuchal lappet distinct; dorsal tubercles globular with black core; venter pubescent; anus dorsal between chaetigers 25-26; body 40 mm long, 7 mm wide, 26 chaetigers).
One specimen (UF 3986), Madang Province, University Road (5.22° S, 145.79° E), 15 m, 12 Nov. 2012, B. Faure, R. Ibik & P.-H. Kuo, coll. (complete, juvenile, markedly bent ventrally after removal of left parapodia of chaetigers 11-16; not dissected to avoid further damage; elytra 2-6 with black spot in insertion area; elytra of median and posterior chaetigers with macrotubercles pale and black; nuchal lappet short; dorsal tubercles globular with black core, better defined intersegmentally; venter pubescent; anus dorsal between chaetigers 25-26; exposed enteron with globular, longer than wide caeca; body 14 mm long, 2.5 mm wide, 26 chaetigers).
Remarks
Hermenia neoverruculosa resembles H. verruculosa because both have dorsal globular tubercles, elytra with spinous macrotubercles and papillae. However, H. neoverruculosa differs from H. verruculosa in that its dorsal tubercles are shorter and densely packed, its venter is smooth, segment 2 is dorsally narrow between the first pair of elytrophores, and projected as a nuchal lappet over the prostomium, notochaetae are small in most parapodia, and neurochaetae have mostly 2 accessory teeth.
Hermenia neoverruculosa had been recorded only in the Indian Ocean, including D’arros Island (Monro, 1924:40), and the Seychelles (Monro, 1939:169). Pettibone (1975) recorded both materials and the features match the species.
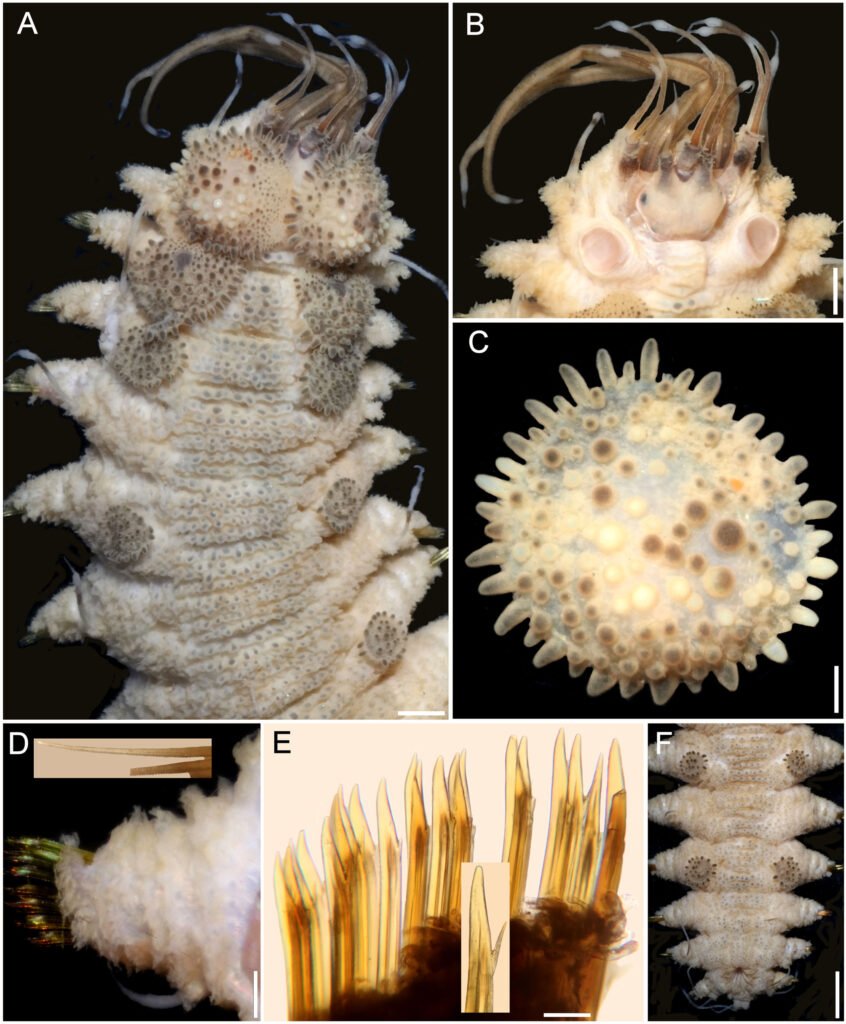
Hermenia chuarae sp. nov.
(Fig. 7)
http://zoobank.org/urn:lsid:zoobank.org:act:59B0411C-59EB-4533-BAC3-5DE3698C850F
Hermenia acantholepis: Chuar et al., 2021: 64-65, Fig. 3G (non Grube, 1876 (Sunda Strait, Indonesia, 448-469 m, on sediment).
Diagnosis. Hermenia with body subrectangular; first and second pair of elytra overlapping; dorsal integument with abundant depressed tubercles, with pale core; segment 2 with nuchal lappet wide; median and posterior elytra with pale and brownish macrotubercles; neurochaetae tridentate.
Description. Holotype (MMMM 000) complete, body depressed, subrectangular, slightly bent laterally, 37 mm long, 6 mm wide, 26 chaetigers. Dorsum with transverse rows of depressed tubercles (Fig. 7A), core pale, of similar size along body; tubercles brownish, darker basally. Venter pale, wrinkled, with abundant filiform papillae.
Prostomium bilobed, about as wide as long, slightly retracted into segment 2; facial tubercle not visible dorsally, pale, with a median globular tubercle. Eyes black; anterior eyes about 2 times as large as posterior ones, directed anteriorly, on the widest part of the prostomium (Fig. 7B); posterior eyes near posterior margin. Antennae, palps, and tentacular cirri directed posteriorly. Median antenna with ceratophore papillate, inserted frontally, 2 times as wide and longer than laterals, ceratostyle about 2 times as long as prostomium, thin, subdistally swollen, swollen region pale, darker along it, tip thin, dark; lateral antennae with ceratostyles 1.5 times as long as prostomium, shorter than median one, with similar pigmentation pattern. Palps tapered, papillate, subdistally with a black ring, tips pale.
Tentacular segment indistinct dorsally, tentaculophores thin, long, with a single short chaeta. Tentacular cirri longer than median antenna, shorter than palps, with a similar pigmentation pattern to the antennae.
Segment 2 narrow with a wide rounded nuchal lappet. First pair of elytrophores reaching bases of tentaculophores. Nephridial papillae not visible along anterior segments, distinct from chaetiger 7, longer along median and posterior segments, often with marginal papillae.
First 3 pairs of elytra larger than the following ones, elytron 3 slightly larger than twice elytron 4. First pair of elytra overlapped dorsally; elytral surface with macrotubercles white and brownish (Fig. 7C), and papillae indistinct; macrotubercles short, globular to elongate, blunt. Elytra of median and posterior segments small, macrotubercles pale or brownish, with abundant papillae along margins.
Parapodia with notopodia reduced to acicular lobe (Fig. 7D), acicular papilla present. Dorsal cirri thin, smooth, short, not reaching neurochaete tips, resembling antennae; cirrophore short, cylindrical. Neuropodia thick, robust, distally with small papillae, and small prechaetal and postchaetal lobes with long papillae; prechaetal lobe larger than postchaetal one. Ventral cirri short, tapered, reaching the lower tip of the neurochaetal lobe.
Notochaetae thin, short, 3-4 laterally denticulate capillaries (Fig. 7D, inset). Neurochaetae thick, upper region short, main fang long, slightly falcate, accessory teeth short, 1/3 as long as main fang (Fig. 7E). Median parapodia with neurochaetae often with 2 accessory teeth, sometimes 1 denticle broken.
Posterior region tapered, truncate (Fig. 7F); pygidium with anus dorsal, anal cirri long, resembling antennae, about as long as last 3 chaetigers.
Taxonomic summary
Type material. Sunda Strait, Indonesia. Holotype (MZB Pol. 300), South Java Deep-Sea Biodiversity Expedition 2018, RV Baruna Jaya VIII, Sta. DW17 (06°07.33’ S, 105°00.76’ E to 06°07.22’ S, 105°00.86’ E), 448-469 m, 26 Mar. 2018.
Distribution. Only known from the Sunda Strait, Indonesia, in bathyal depths.
Etymology. This species is named after Miss Cheah Hoay Chuar. She is a polychaete taxonomist at the National University of Singapore, in recognition of her involvement in the South Java Deep Sea Biodiversity Expedition 2018, and because she kindly allowed us to study the holotype for this species.
Remarks
Hermenia chuarae sp. nov. resembles H. neoverruculosa, originally described from the Cargados Carajos Archipelago, Republic of Mauritius, Indian Ocean. Both species have a dorsum grayish with transverse rows of tubercles and elytral macrotubercles globular, non-L-shaped. However, they differ because H. chuarae has dorsal tubercles depressed, without black cores, and the median and posterior elytra have pale and brownish macrotubercles, whereas in H. neoverruculosa the dorsal tubercles are globular with black cores, and its median and posterior elytra have black macrotubercles.
On the other hand, the holotype of H. chuarae is the deepest record for any Hermenia species, being collected in sediments at 448-469 m depth.
Hermenia mezianei sp. nov.
(Fig. 8)
http://zoobank.org/urn:lsid:zoobank.org:act:500F2A6D-2A63-44F5-8DAB-EDE4646B7DCB
Hermenia acantholepis: Fauvel, 1935: 281, 286 (Vietnam, Pacific Ocean); Fauvel, 1939: 256 (Vietnam, Pacific Ocean); Dawydoff, 1952: 90 (non Grube, 1876; Vietnam, Pacific Ocean).
Diagnosis. Hermenia with body blunt fusiform; only first pair of elytra overlapping, first 3 pairs larger than following ones; median elytra oval barely covering adjacent segments; elytral tubercles globular, with a basal hump; dorsal integument white, barely rugose anteriorly, with thin papillae; segment 2 barely projected over prostomium as a small nuchal lappet; neurochaetae 2 accessory teeth.
Description. Holotype (MNHN A398) complete, dorsum wrinkled, almost smooth, a few sparse tubercles and larger papillae anteriorly (Fig. 8A); left elytra 1, right elytra 1, 2, and right parapodia of chaetigers 2, 14, and 15 previously dissected (kept in container), venter smooth, without papillae; body depressed, 13.5 mm long, 4.5 mm wide, 26 chaetigers.
Prostomium partially retracted into following segment, subhexagonal, wider than long (Fig. 8B); facial tubercle not visible dorsally, low; 2 pairs of black eyes of similar size. Median antenna with ceratophore 2 times as wide and slightly longer than laterals, ceratostyle short, tapered (probably in regeneration); right lateral ceratostyle smooth, tapered, subdistally wider, longer than palps. Palps barely papillate.
Tentacular segment not visible dorsally, tentaculophores thick, without chaetae; right tentacular cirri asymmetrical, ventral ceratostyle one 2 times as long as dorsal one (probably in regeneration). Segment 2 was previously slightly dissected, with a short nuchal lappet. Elytrophores not covering tentaculopohores.
Elytra small, not overlapped laterally nor covering dorsum: first 3 elytra larger than median ones, elytra 1 and 2 of similar size, elytron 3 about half their size, elytron 4 and following ones slightly smaller than elytron 3, oval, wider than long, barely covering adjacent segments. Elytra surface with tubercles and sparse filiform papillae (Fig. 8C). Tubercles abundant, ovoid, spinous, some basally bulbous, most globular, projected beyond elytral margins; marginal tubercles progressively smaller in median and posterior segments, pale or brownish.
Parapodia with notopodia reduced, blunt conical ridge over neuropodia (Fig. 8D). Neuropodia thick, surface with transverse wrinkled rows, prechaetal and postchaetal lobes truncate, of similar size, with margin papillate.
Dorsal cirri long, reaching upper neurochaetae, subdistally swollen, brownish, tips long filiform, resembling antennae and tentacular cirri. Cirrophore very short, cylindrical. Ventral cirri short, tapered, surface smooth. Nephridial papillae globular, short, along chaetigers 6-25.

Notochaetae short, scarce, fragile, tips broken (Fig. 8D, inset), with impurities, marginally denticulate. Neurochaetae thick, smooth, upper region short; tips thick, slightly falcate, with 2 short, subdistal accessory teeth (Fig. 8E).
Posterior region tapered, blunt (Fig. 8F); last pair of elytra of similar size as preceding ones, pygidium with anus dorsal between chaetigers 25 and 26, anal cirri missing.
Taxonomic summary
Type material. Vietnam. Holotype (MNHN A398), Hón Lön Island (Honlohé, Ile Mamelles), By of Nha Trang, C. Dawydoff, coll. (no further data).
Distribution. Only known from Vietnam and Futuna Island (South Pacific Ocean), in substrates in platform-shelf depths.
Etymology. The specific epithet is after Dr. Tarik Meziane, curator of the Annelid collection in the Muséum National d’Histoire Naturelle, Paris, in recognition of the support he has kindly provided to our research projects.
Additional material. Futuna Island, Western Pacific, One specimen (MNHN IA 2023-45), Cruise MUSORSTOM 7, RV Alis, Sta. CP517 (14°13’23.99’’ S, 178°10’24.02’’ W), 223-235 m, 12 May 1992, P. Bouchet et al., coll. (partially dehydrated, markedly bent ventrally, integument tuberculate; first 3 pairs of elytra larger than following ones, third elytra about 2 times as large as fourth one; median and posterior elytra circular to oval, slightly wider than long, barely covering segment; last pair of elytra as large as previous one; not measured to avoid further damage).
Two specimens (MNHN IA 2023-48), Cruise MUSORSTOM 7, RV Alis, Sta. CP498 (14°18’54’’ S, 178°03’05.99’’ W), 105-160 m, 10 May 1992, P. Bouchet et al., coll. (integument tuberculate; first 3 pairs of elytra larger than following ones, third elytra about 2 times as large as fourth one; median and posterior elytra circular to oval, slightly wider than long, barely covering segment; last pair of elytra slightly larger than precedent one; body 18-28 mm long, 5.5-6.0 mm wide, 26 chaetigers).
Remarks
Hermenia mezianei sp. nov. resembles H. acantholepis restricted, by having a whitish, almost smooth integument, first 3 pairs of elytra larger than the following ones, each with marginal macrotubercles basally swollen, and trifid neurochaetae. Their main differences are the shape and size of the median and posterior elytra, the distribution of macrotubercles in the first elytra, and the type of macrotubercles in the median and posterior elytra. First, in H. mezianei the median and posterior elytra are oval, wider than long, barely covering adjacent segments, whereas in H. acantholepis they are circular and cover at least half of the adjacent segments. Second, the first elytra of H. mezianei have the anterior inner area with round macrotubercles, whereas there are no macrotubercles in the same area in H. acantholepis, only microtubercles. Third, macrotubercles in median and posterior elytra in H. mezianei are short, globular, mostly pale, barely projected from the elytral margin, whereas in H. acantholepis they are digitate, almost all brownish, markedly projected from the elytral margin.
Hermenia treadwelli sp. nov.
(Figs. 9, 10)
http://zoobank.org/urn:lsid:zoobank.org:act:9588D5E3-1895-4086-AD94-A650503CB7F7
Hermenia verruculosa: Treadwell, 1911: 9-11, Figs 23-26 (partim, nonGrube & Örsted in Grube, 1856; Dry Tortugas, Florida in dead coral); Pettibone, 1975: 235-238, Figs 1, 2 (syn., partim; only his specimens completely white along anterior chaetigers.
Diagnosis. Hermenia with body fusiform; segment 2 with short nuchal lappet; first pair of elytra bigger, not overlapping, other elytra tiny; dorsal integument with abundant dorsal tubercles, paler ones forming an inverted T-shaped spot in segments 5 or 5 anterior segments white, with pale tubercles; segment 2 with short projection over prostomium as a nuchal lappet; neurochaetae bidentate.
Description. Holotype (ECOSUR 1396) with body robust, depressed, fusiform, widest medially, 24 mm long, 6.5 mm wide, 26 segments. Dorsum covered with globular tubercles of different sizes, some larger than the others, larger in anterior and posterior segments, tubercles dark brown in anterior segments, paler towards posterior part in cirrigerous segments, giving an appearance of banding of dark segments alternating with pale ones. First 5 segments almost completely white (Fig. 9A). Venter with thick digitiform papillae.
Prostomium bilobed (Fig. 9B), retracted into the second segment. Two pairs of dark eyes, anterior pair on the widest part of the prostomium; posterior eyes covered by second segment. Palps robust with papillae, tapered, tips filiform (left palp lost). Three antennae, median antennae with ceratophore inserted frontally on prostomial lobe, about 2 times as wide as laterals, cylindrical, darker than prostomium, ceratostyles lost. Pharynx not everted.
First pair of elytra largest, round (Fig. 9C), covering prostomium, following elytra smaller; first pair of elytra with papillae digitiform, short, thick on the surface and along margin; elytral surface with many macrotubercles, present on elytrophore mark, larger towards margin. Elytra of median and posterior segments with abundant digitiform papillae on surface and along margin; macrotubercles thick, hemispherical, spinous.
Tentacular segment not visible dorsally; tentaculophores short, without chaetae; tentacular cirri thick, similar in shape and length as the median antenna. Segment 2 narrow between the first pairs of elytrophores, projected on the prostomium as a nuchal lappet. First pair of elytrophores expanded.
Parapodia biramous, truncate, dorsal surface with rows of globular tubercles (Fig, 9D). Notopodia short, acicular ridge covered with 1 blunt papilla (Fig. 10D, inset left). Neuropodia thick with prechaetal and postchaetal lobes reduced, each with abundant marginal papillae. Cirrigerous segments with dorsal cirri short, not surpassing neuropodia tips, surface smooth, similar in shape to antennae; cirrophore short, thick, with papillae; ventral cirri thin, short. Nephridial papillae visible from segment 7, thick, and becoming larger on posterior segments.
Notochaetae absent. Neurochaetae of median and posterior segments thick (Fig. 9D, insets above), upper region short with a long tooth, slightly curved, and a secondary straight denticle distant from each other. Parapodia of the second segment with neurochaetae thinner, of 2 kinds, some with long spines, tips entire, others with spines only subdistally.
Posterior region pale, truncate (Fig. 9E); last pair of elytra as large as preceding ones, pygidum terminal, dorsal anus, anal cirri lost.
Variation. Other specimens have first elytra pale, and an anterior, inverted triangle spot along the dorsum of the chaetigers 3-5 (Fig. 10A). The prostomium retains dark-reddish pigmentation along ceratophores, and the median antenna ceratostyle, palps, and tentacular cirri are brownish, with pale swollen areas (Fig. 10B). The nuchal lappet is better defined as well.
The first elytron has globular macrotubercles, mostly brownish, with abundant marginal papillae and pale microtubercles along its surface (Fig. 10C). Parapodia biramous (Fig. 10D) with barely projected notopodia (Fig. 10D, left inset), and dorsal cirri with conical cirrophore (Fig. 10D, upper inset). Notochaetae broken and lost. Neurochaetae abundant, brownish (Fig. 10E), with 1 subdistal accessory denticle (Fig. 10E, insets).
Posterior region banded (Fig. 10F), elytrigerous chaetigers with dorsal surface darker, cirrigerous chaetigers paler; pygidium with anus dorsal, between chaetigers 25-26, anal cirri lost.
Taxonomic summary
Type material. Caribbean Sea. Holotype (ECOSUR 1396), Chinchorro Bank, Cayo Norte, R/V Edwin Link, Sta. 2774 (18º45.63’ N, 87º15.84’ W), 55 m, August, 1990 E. Escobar, L. Soto, J.L. Villalobos, coll.
Distribution. Western Tropical Atlantic (Bahamas to Venezuela), in shallow mixed bottoms.
Etymology. The species is named to honor the late Dr. Aaron Treadwell in recognition of his contributions to the knowledge of different families of polychaetes.
Additional material. Northwestern Atlantic. One specimen (USNM 3296), USFCS Albatross, Sta. 2246 (39°56’45” N, 70°20’30” W), off New Jersey, U.S.A., Sta. 188, 220 m, 26 Sep. 1884 (posterior region bent ventrally; dorsum of chaetigers 2-5 with a discontinuous inverted T-shaped white spot; body 22 mm long, 5.5 mm wide, 26 chaetigers).
Bahamas. One specimen (USNM 16492), Holding Key, Andros Island, 13 May 1912, P. Bartsch, coll. (slightly bent laterally; chaetigers 2-5 with dorsum almost completely white; body 20 mm long, 5 mm wide, 26 chaetigers).
One specimen (USNM 46921), outer reef, point north of Clarencetown, Long Island, 15 May 1970, C. Riser, coll. (chaetigers 4-5 with a wide, inverted T-shaped white band, interrupted laterally; median and posterior nephridial lobes brownish; body 28.5 mm long, 5.5 mm wide, 26 chaetigers).
Florida. One specimen (USNM 17722), Tortugas, 1914, A.L. Treadwell, coll. (dorsum brownish, with inverted T-shaped wide band in chaetigers 4-5; body nephridial lobes from chaetiger 8, progressively larger posteriorly; 34 mm long, 7 mm wide, 26 chaetigers).
Caribbean Sea. One specimen (ECOSUR 9), Majagual, Quintana Roo, México, 6 Jun. 1998, M.A. Ruiz-Zárate, coll. (juvenile; slightly twisted; no dissected to avoid further damage; without nuchal lappet; anterior region with inverted T-shaped white spot along chaetigers 4-5; anus in chaetiger 26; body 11 mm long, 3 mm wide, 26 chaetigers).
One specimen (ECOSUR 1305), Majagual, Quintana Roo, México, 6 Jun. 1998, M.A. Ruíz-Zárate, coll. (juvenile, complete; bent ventrally; chaetigers 1-5 completely white; nuchal lappet distinct; anus terminal in chaetiger 26; body 11 mm long, 3.5 mm wide, 26 chaetigers).
One specimen (ECOSUR 1312), Xcacel, Quintana Roo, México, 17 Apr. 1996, S.I. Salazar-Vallejo & L.F. Carrera-Parra, coll. (complete; bent ventrally; pharynx exposed, 10 pairs of marginal papillae; chaetigers 1-5 completely white; nuchal lappet short; anus terminal in chaetiger 26; body 18 mm long, 5 mm wide, 26 chaetigers).
One specimen (ECOSUR 1318), Venezuela, Margarita Island, Los Testigos, seagrasses, 6 Jun. 1961 (partially dehydrated; anterior region with inverted triangle white spot along chaetigers 4-5; nuchal lappet distinct; anus in chaetiger 26; body 19 mm long, 5 mm wide, 26 chaetigers).
Two specimens (ECOSUR 1394), Punta Nizuc, Cancún, Quintana Roo, coral rocks, 1 Sep. 1997, S.I. Salazar-Vallejo, L.F. Carrera-Parra & M.A. Ruiz-Zárate, coll. (complete, fusiform; first 5 chaetigers almost completely white; nuchal lappet short; anus terminal in chaetiger 26; body 20-27 mm long, 5.5-7.5 mm wide, chaetigers).
One specimen (ECOSUR 1395), Majagual, Quintana Roo, reef lagoon, rocks, 18 Mar. 2002, S.I. Salazar-Vallejo, coll. (complete, fusiform; first 5 chaetigers almost completely white; nuchal lappet distinct; anus terminal in chaetiger 26; body 28.5 mm long, 7 mm wide, chaetigers).
One specimen (ECOSUR 2429), Punta Herradura, Quintana Roo, México, 28 Oct. 1997, P. Salazar-Silva, coll. (bent laterally; first right elytron and left parapodium of chaetiger 5 previously removed, kept in container; anterior region with inverted T-shaped white spot along chaetigers 4-5; anus prolapsed, in chaetiger 26; body 21 mm long, 5.5 mm wide, 26 chaetigers).
One specimen (LACM 14762), British Virgin Islands, Guana Island, Beef Island, long white beach at far west end of airport property, (18.448° N, 64.541° W), coral rubble, 1-2 m, snorkel, hand, 23 Jul 2000. T. Zimmerman, T. Haney, R. Ware, D. Cadien, coll. (barely bent ventrally, with salt particles adsorbed on body; chaetigers 4-5 with an inverted T-shaped white band; body 26.5 mm long, 7 mm wide, 26 chaetigers).
One specimen (LACM 147883), Jamaica, Saint Ann Bay, Bull Reef (18.735° N, 77.289° W), coral rubble and algae, 8 m, 2 Jun. 2006, K. Rawlinson, M. Bolanos, A. DuPont, A. Allan, J. Dunn & L. Harris, coll. (slightly bent ventrally, pharynx partially exposed; chaetigers 2-5 completely white dorsally; body 14.5 mm long, 3.5 mm wide, 26 chaetigers).
Three specimens (LACM 147910), British Virgin Islands, Guana Island, White Bay, ARMS (18.3° N, 64.37° W), 9 m, 15 Jul. 2000, J. Zimmerman, J. Martin & R. Ware, coll. (smaller ones almost colorless, largest bent ventrally, with an inverted whitish T-shaped spot along anterior chaetigers; body 7-26 mm long, 2.0-6.5 mm wide, 26 chaetigers).
One specimen (UMML 22.729b), University of Miami, Cruise P7006, Hispanola and Jamaica, RV Pillsbury, Sta. 1198 (17°49.4’ N, 76°12.3’ W), 29-37 m, 4 Jul. 1970 (bent ventrally; smaller one with inverted T-shaped white spot in chaetigers 2-5, anus terminal in chaetiger 26; body 15 mm long, 5 mm wide, 26 chaetigers).
One specimen (UMML P341), University of Miami, Cruise 6607, Panama to Venezuela, R/V Pillsbury, Sta. 341 (09°02’ N, 77°02’ W), 44 m, 9 Jul. 1966 (anterior region with an inverted T-shaped spot in chaetigers 2-5; first left elytron and left parapodia of chaetigers 3, 4, 13 previously removed (elytron and 1 parapodium kept in container); anus in chaetiger 26; body 15 mm long, 5 mm wide, 26 chaetigers).
Three specimens (UMML P1148), University of Miami, Cruise P7006, Hispanola and Jamaica, RV Pillsbury, Sta. 1148 (20°00’ N, 71°41’ W), 38 m, 1 Jan. 1970 (anterior region with inverted T-shaped spot in chaetigers 2-5; 1 specimen with anterior end dissected, several parapodia and elytra previously remove, kept in container; nuchal lappet distinct; dorsal cirri completely pale; body 21-24 mm long, 6-7 mm wide, 26 chaetigers).
One specimen (UMML P1330), RV Pillsbury, Cruise P7101, Central America, off Nicaragua, Sta. 1330 (11°51’ N, 83°27’ W), 24 m, 28 Jan. 1971 (soft, bent ventrally; chaetigers 4-5 with an inverted triangle white spot; nuchal lappet indistinct; anus terminal in chaetiger 26; body 21 mm long, 6 mm wide, 26 chaetigers).
One specimen (USNM 951), off Colon, Panama, USFSS Albatross, Sta. 2146 (9°32’00” N, 79°54’30” W), 61 m, 2 Apr. 1884, 61 m. (bent ventrally and laterally; chaetigers 4-5 with an inverted T-shaped dorsal white band; body 26 mm long, 7.5 mm wide, 26 chaetigers).
One specimen (USNM 20489), Old Providence, Colombia, coll. W.L. Schmitt, coll., 6 Aug. 1938, shore, reef and tide pool. (bent ventrally, chaetigers 2-5 with an inverted T-shaped white band; body 22 mm long, 4 mm wide, 27 chaetigers).
One specimen (USNM 46912) St. James Island, near St. Thomas, 4.5 m, 10 Jul. 1915, C.R. Shoemaker, coll. (body slightly bent laterally; dorsum of chaetigers 4-5 with a discontinuous inverted T-shaped white spot; anterior chaetigers with notochaetae; body 21 mm long, 5.5 mm wide, 26 chaetigers).
One specimen (USNM 46915), Barbuda, around Spanish Point, in beach wrack, fossil coral and live Porites, Sta. 112-58, 28 Apr. 1958, W. L. Schmitt, coll. (chaetigers 4-5 with a wide, inverted T-shaped white band; left parapodia of chaetigers 22-23 broken; breaking in posterior end; body 25 mm long, 6 mm wide, 27 chaetigers).
One specimen (USNM 46919), off Loggerhead Key, Dry Tortugas, Florida, 6 m, matrix of brain coral, 19 Aug. 19 1966, R. F Cressey, coll., & donor, (only 1 of the 2 specimens identified by M. H. Pettibone: wider slightly bent ventrally, thinner with first few chaetigers almost completely white, wider with 3 white spots in chaetiger 6 and a median on in chaetigers 4-5; body 16-18 mm long, 5.0-5.5 mm wide, 26 chaetigers).
One specimen (USNM 46922), U.S. Virgin Islands, 90 m, Sep. 1970, J. Clark, coll. (chaetigers 4-5 with a wide, inverted T-shaped white band; many chaetae broken; posterior end bent ventrally; median and posterior nephridial lobes brownish; body 21.5 mm long, 6.5 mm wide, 26 chaetigers).
No location. USNM 34285 (2), w/o field data, 11 Jan. 1966. (soft, barely pigmented, 1 with a dorsal white spot, the other with a discontinuous inverted T-shaped white spot in chaetigers 4-5; smaller specimen with several left posterior parapodia previously removed, some in container; body 24-28 mm long, 6-7 mm wide, 26 chaetigers).
Remarks
Hermenia treadwelli sp. nov. is distinguished by having a body fusiform, depressed, wider medially, venter wrinkled, with papillae thick, abundant; nuchal lappet thick, shorter than in H. neoverruculosa. Dorsum with globular tubercles of different sizes, some prominent giving a crowded appearance, parapodia short; from the second pair of elytrophores, elytra smaller, fleshy, barely covering elytrophores, firmly attached.
Hermenia treadwelli differs from H. verruculosa by having a body depressed, fusiform, venter with papillae thick, long, segment 2 with a well-developed nuchal lappet, on anterior part of the dorsum with a T-shaped spot with pale tubercles, extended along almost the whole segment 6, and middorsal areas of segment 5, whereas in H. verruculosa the body is subrectangular, venter with papillae thin, short, without nuchal lappet on prostomium, and the small spot of unpigmented tubercles is small and extended middorsally along segments 5-6.
Further, H. treadwelli differs from H. neoverruculosa because its nuchal lappet is shorter, neurochaetae only have 1 accessory tooth, the venter has papillae instead of being smooth, and neurochaetae with 2 accessory teeth.
After the body shape, being depressed, and retracted prostomium, venter with thick papillae, H. treadwelli body indicates a species with reduced mobility in comparison with H. verruculosa or H. neoverruculosa.
Hermenia wehei sp. nov.
(Fig. 11)
http://zoobank.org/urn:lsid:zoobank.org:act:08AE4922-3010-4327-B8AA-9753DD0AE1D1
Hermenia verruculosa: Wehe, 2006: 80-81, Pl. 1, Fig. d (non Grube & Örsted in Grube, 1856; Socotra Island, Indian Ocean, 8-10 m).
Diagnosis. Hermenia with body subrectangular; first pair of elytra bigger, cover prostomium, remaining elytra tiny; dorsal integument with abundant globular tubercles, without larger ones along middline; neurochaetae with 2 o 3 accessory teeth.
Description. Holotype (SMF 136089) complete, bent ventrally (Fig. 11A), slightly wider anteriorly, 26.5 mm long, 18 mm wide, 26 chaetigers. Dorsum almost without pigmentation (pale with elytrigerous segments brownish, cirrigerous whitish; elytra and a few middorsal tubercles brownish); dorsal tubercles medium sized, larger middorsal tubercles missing. Venter almost smooth.
Prostomium markedly retracted into segment 2 (Fig. 11B); facial tubercle not seen; eyes not seen. Median ceratophore slightly wider and longer than laterals; left lateral antenna present, almost half as long as palps. Palps bent ventrally, with abundant papillae in longitudinal rows.
Tentacular segment indistinct dorsally. Tentaculophores thick, cylindrical, without chaetae. Tentacular cirri subequal, almost as long as palps. Second segment narrow middorsal, without large globular tubercles, without nuchal lappet; elytrophores not expanded anteriorly; right ventral cirrus lost, left one about 6 times longer than the following one.
First pair of elytra largest, pale, completely cover prostomium; elytral surface with abundant macrotubercles markedly surpassing elytral margins (Fig. 11C), and smaller microtubercles; macrotubercles globular, longer than wide, surface spinulose, present along all elytral surface, some papillae present along inner margin. Following elytra of similar size, circular, not removed to avoid further damage, with macro- and microtubercles, mostly globular.
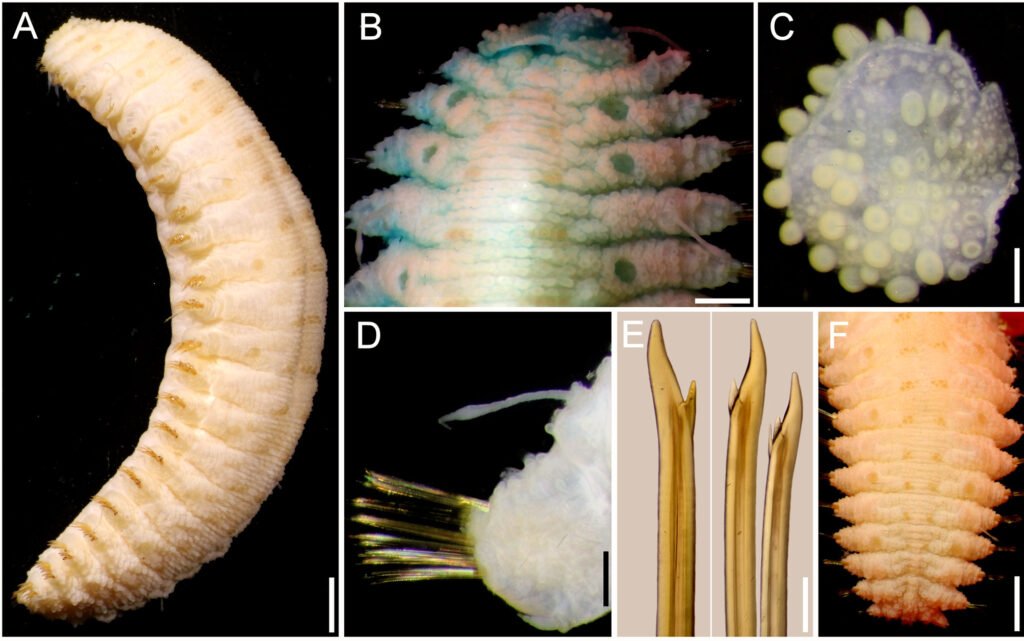
Parapodia short, 1/4 as long as body width; dorsally with transverse rows of globular tubercles along notopodia, remaining surface with papillae, ventrally with transverse pleats and scattered papillae. Notopodia reduced, acicular lobe small, with an apical papilla. Neuropodia truncate, dorsally furrowed, prechaetal lobe slightly larger than postchaetal one, with some distal papillae.
Cirrigerous segments with cylindrical, thick, short cirrophores, cirrostyles smooth, long, almost smooth, reaching the neuropodial tip (Fig. 11D). Nephridial papillae low, blunt, from chaetiger 12, continued to chaetiger 25, progressively larger.
Neurochaetae amber color. Most body segments with 2-3 notochaetae, curved, marginally denticulate. Neurochaetae decreasing in size and width ventrally, most with 2 accessory teeth, on lower position (Fig. 11E).
Posterior region tapered, truncate; pygidium with anus terminal, anal cirri lost (Fig. 11F).
Taxonomic summary
Type material. Arabian Sea. Holotype (SMF 136089, Socotra Island, Sta. 726, 8-10 m, 9 Apr. 2000, T. Wehe, coll.
Distribution. Known only from Socotra Island, Arabian Sea, and from Saya de Malha Bank, Indian Ocean; in subtidal substrates (8-26 m).
Etymology. The specific epithet is after Dr. Thomas Wehe, in recognition of his relevant publications on scaleworms, and especially because he collected the holotype.
Additional material: Indian Ocean, Saya de Malha Bank.One specimen (MNHN IA 2017-4879), Saya Expedition, RV Agulhas II, Sta. YS08 (09°41.9’ S, 60°51’ E), 26 m, 8 Nov. 2022, S. Hourdez, coll. (complete, bent ventrally, with ill-defined transverse bands in elytrigerous segments; first elytra pair markedly larger than following ones, barely covering prostomial sides; pharynx everted, 4 mm long, with 10 pairs of terminal papillae; right parapodium of chaetiger 15 removed for observation; neurochaetae with a single accessory denticle (rarely double); body 19 mm long, 5 mm wide, 26 chaetigers).
One specimen (MNHN 2021-727), Saya Expedition, RV Agulhas II, Sta. YS08 (16°50’ S, 59°31.3’ E), 17 m, 20 Nov. 2022, S. Hourdez, coll. (complete, bent ventrally with ill-defined transverse bands in elytrigerous segments; first elytra pair markedly larger than following ones, barely covering prostomial sides; left parapodia of chaetigers 16-19 removed for molecular studies; body 23 mm long, 6.5 mm wide, 26 chaetigers).
Remarks
Hermenia wehei sp. nov. belongs to the group of species (H. verruculosa, H. treadwelli) having only the first pair of elytra markedly larger than the following ones. However, H. wehei separates from the other species regarding differences in the first elytra, the development of middorsal tubercles, and in neurochaetal tips. Thus, H. wehei has the first elytra with macrotubercles clearly projected beyond the margin (against barely projected), medium-sized middorsal tubercles (against having larger ones middorsally), and its neurochaetae are bidentate or tridentate (against only bidentate in the other species).
Key to species of Hermenia Grube & Örsted in Grube, 1856
1 First 2-3 anterior pairs of elytra markedly larger than posterior ones; neurochaetae of median segments with 2 accessory teeth
………………………………………………………………………………2
– Only first pair of elytra markedly larger than posterior ones; neurochaetae of median segments with 1 accessory denticle, rarely with 2 accessory teeth
………………………………………………………………………………5
2 (1) Dorsum whitish, papillate or barely tuberculate; elytral macrotubercles globular, basally swollen or L-shaped (inner projection smaller)
………………………………………………………………………………3
– Dorsum grayish, clearly tuberculate; tubercles arranged in transverse series; elytral macrotubercles globular, no L-shaped
………………………………………………………………………………4
3 (2) Median and posterior elytra covering up to half of adjacent segments, elytra round, last one slightly larger than previous one
………………………………………………………………………………H. acantholepis (Grube, 1876) Philippines
– Median and posterior elytra barely covering adjacent segments, elytra oval to circular, last elytra as large as previous one
………………………………………………………………………………H. mezianei sp. nov. Vietnam
4 (2) Dorsum with rows of globular tubercles, each with blackish core; median and posterior elytra with black macrotubercles
………………………………………………………………………………H. neoverruculosa Pettibone, 1975 Cargados Carajos, Indian Ocean
– Dorsum with rows of depressed tubercles, each with pale core; median and posterior elytra with pale and brownish macrotubercles
………………………………………………………………………………H. chuarae sp. nov. Indonesia
5 (1) Neurochaetae with a single accessory tooth; middorsal surface with large globular tubercles; first left elytron with macrotubercles barely projected beyond margin
………………………………………………………………………………6
– Neurochaetae with 1-2 accessory teeth; middorsal surface without large globular tubercles; first left elytron with macrotubercles distinctly projected beyond margin
………………………………………………………………………………H. wehei sp. nov. Arabian Sea
6 (5) Segment 2 with nuchal lappet over prostomium; dorsal cirrostyle of median segments with swollen area brownish (fresh specimens with chaetigers 1-5 almost completely white, or with a large white inverted triangular spot over the dorsum of the chaetigers 3-5)
………………………………………………………………………………H. treadwelli sp. nov. Grand Caribbean Sea
– Segment 2 without nuchal lappet over prostomium; dorsal cirrostyle of median segments with swollen area whitish (fresh specimens with a middorsal white rectangular spot, or dorsum completely dark along chaetigers 5-6, sometimes separated by brownish area into 3 spots)
………………………………………………………………………………H. verruculosa Grube & Örsted in Grube, 1856 U.S. Virgin Islands, Caribbean Sea
Parahermenia gen. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:5604018E-9BC0-4D3C-908F-1E355BAEFE7A
Diagnosis. Lepidonotinae with short body; integument papillate, venter papillate. Body with 26 segments, 12 pairs of elytra on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23. Prostomium bilobed, with 2 pairs of eyes. Elytra with globular macrotubercles and microtubercles, abundant papillae, fimbriae very long; elytra of similar size along body, overlapping laterally, not dorsally. Tentacular segment with few notochaetae and bulbous facial tubercle. Parapodia subbiramous, notopodia small, neuropodia large. Notochaetae long, slender, finely spinous. Neurochaetae stout, falcate, with 1-2 large accessory teeth. Dorsal cirri with cirrophores cylindrical, cirrostyles short. Ventral cirri short, subulate. Pharynx with 2 pairs of jaws and 9 pairs of marginal papillae. Nephridial papillae short, cylindrical, from chaetigers 6-8.
Taxonomic summary
Type species. Lepidonotus hermenioides Amoureux, 1974.
Distribution. Parahermenia includes 2 species found in shallow water sediments, 1 from Madagascar, the other from the Philippines.
Etymology. Parahermenia is made by combining the stem genus name Hermenia with the Greek prefix para-, meaning “besides, nearby” (Brown 1954: 587), and this new name indicates the affinities to the older genus group name. Gender: Feminine.
Remarks
Pettibone (1977:40) noted that there are 3 genera with long filiform papillae on the distal end of neuropodia. She proposed 2 new genera, Lepidonopsis Pettibone, 1977 and Parahalosydnopsis Pettibone, 1977, which, together with Halosydnopsis Uschakov & Wu, 1959, were the only genera provided with filiform papillae. The number of pairs of elytra and neurochaetal tips separates these 3 genera because Lepidonopsis has 12 pairs and bidentate neurochaetae, Halosydnopsis has 17 and unidentate neurochaetae, and Parahalosydnopsis has about 30 pairs and unidentate neurochaetae. These 3 genera have small denticles in series along the pectinate area.
Lepidonotus hermenioides does not belong in Lepidonopsis although it has 12 pairs of elytra, because each elytron is provided with very long fimbria, only 2 macrotubercles in the insertion area and the elytra do not overlaped dorsally, whereas in Lepidonopsis the elytra overlap covering the body, have short fimbriae and different types of microtubercles (Salazar-Silva & Carrera-Parra, 2014). On the other hand, L. hermenioides does not fall within Hermenia despite sharing a papillate integument, and neurochaetae with 1-2 accessory teeth. The main differences are in the elytra. In L. hermenioides elytra are of similar size along the body, overlapping laterally but not dorsally, and their margin has abundant long fimbriae, whereas in the species of Hermenia median and posterior segments have elytra that do not overlap laterally or dorsally, and fimbriae are minute.
Amoureux (1974) avoided proposing a new genus because he only had a single specimen, and it was regarded as a very small juvenile. He might have suspected some ontogenetic modifications that would complicate separating the new group. We agree this and the new species described below include small specimens; however, after the study of many specimens of different size, we do not find evidence that elytra would be so drastically modified such that they would become progressively smaller during growth, or that the elytral margins would be modified from having very long fimbriae to becoming progressively reduced to become minute.
Parahermenia hermenioidea (Amoureux, 1974) comb. nov.
(Fig. 12)
Lepidonotus hermenioides Amoureux, 1974: 131, Fig. 1.
Diagnosis. Parahermenia with body papillate, elytra with long filamentous papillae, without macrotubercles or spines, margin fimbriate; neurochaetae with 2-3 accessory large teeth.
Description. Holotype (MNHN POLY TYPE 1275) complete, brownish, with abundant papillae along body (Fig. 12A); pharynx fully exposed; elytra paler with long fimbriae, and filaments on elytral surface; first pair of elytra on site, right elytra 10 and 12 on site; right parapodia of chaetigers 10, 12, 14, and left parapodia of chaetigers 13 and 14 previously removed (1 elytron and 1 parapodium in container). Body depressed, wider anteriorly, 3.5 mm long (pharynx 0.8 mm long), 1.2 mm wide (without chaetae), 23 chaetigers.
Prostomium oval, as long as wide; only left lateral antenna, ceratostyle on site, short, all other lost (Fig. 12B); median antenna ceratophore about twice wider than laterals. Palps lost. Eyes indistinct. Pharynx cylindrical, grayish, with 9 pairs of marginal papillae.
Facial tubercle globular, small. Tentacular segment indistinct dorsally; second segment chaetiger without nuchal lappet, dorsum without tubercles; tentacular cirri present on left side, 4-5 times longer than left antenna, subdistally swollen, with 4-5 chaetae.
Ventral cirri of chaetiger 2 are about 3-4 times longer than the following ones, surpassing neurochaetal tips. Nephridial lobes barely developed, not visible.
Elytra oval, with 2 globular macrotubercles in the insertion area, each with small conical papillae; microtubercles conical over elytral surface, and long filaments on surface, margin with long fimbriae, filaments 1/2-1/3 elytral length (Fig. 12C).
Parapodia biramous. Notopodia reduced, low round lobes. Dorsal cirri resembling tentacular cirri, barely swollen subdistally. Notochaetae thin, with a single series of fine denticles (Fig. 12D).
Neuropodia larger, prechaetal and postchaetal lobes blunt, papillate. Neurochaetae barely swollen subdistally, unidentate, with 2-3 large subdistal accessory teeth (Fig. 12E).
Posterior region truncate; pygidum with anus terminal, anal cirri tapered, resembling dorsal cirri.
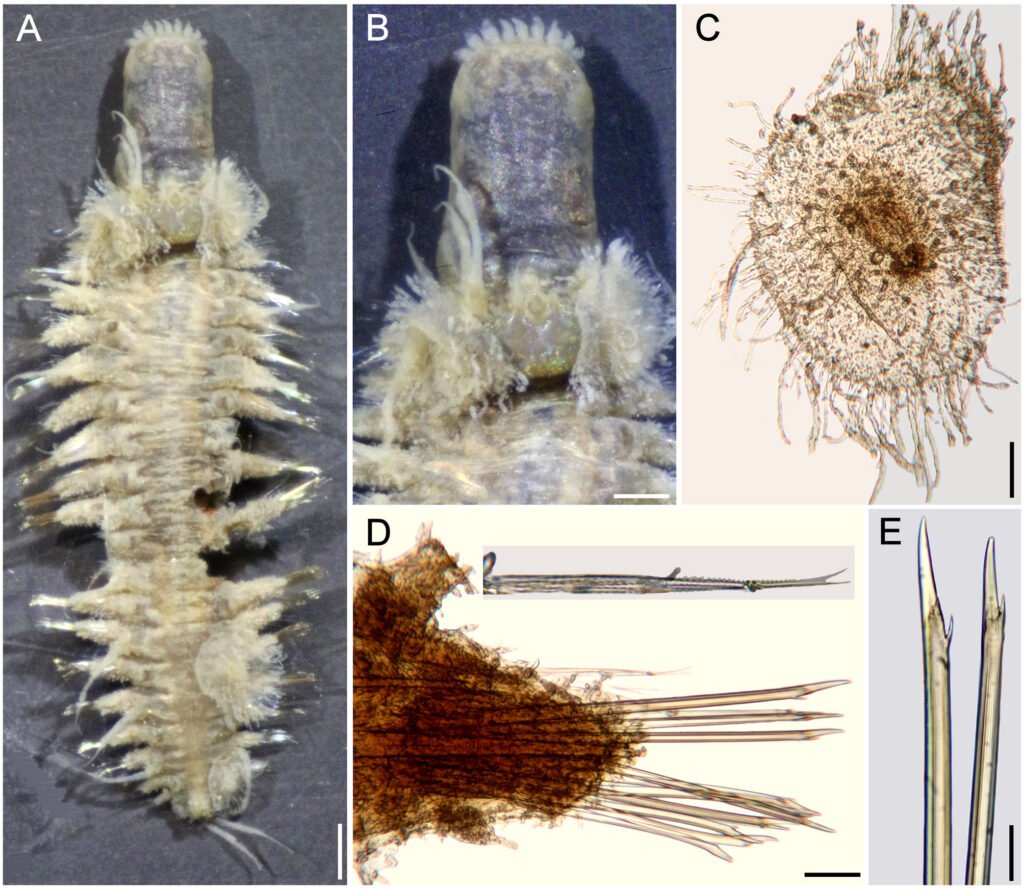
Taxonomic summary
Type material. Madagascar. Holotype of Lepidonotus hermenioides Amoureux, 1974 (MNHN POLY TYPE 1275), Nosy Tanikely, Madagascar, comb-dredge (faubertage) 1, 15 m, 6 Apr. 1960, G. Cherbonnier, leg.
Distribution. Only known from a single locality in Madagascar, in shallow rocky bottoms.
Remarks
Parahermenia hermenioidea (Amoureux, 1974) comb. nov. needed an alteration of the specific name for matching the genus gender. This is regarded as an adjective and, after Brown (1954: 483), the suffix –oid, –oides, –odes, –oideus, –a, –um, are derived from the Greek suffix –eides meaning “like, resembling, having the form of”. He also indicated that –oideus, –a, –um have their gender indicated by the adjectival endings –us, –a, –um.
Amoureux (1974) noted that the neurochaetae of Lepidonotus hermenioides resembles those present in Hermenia, and this explains the specific name. The generic affinities are problematic because L. hermenioides does not match Hermenia by having median and posterior elytra larger, with long fimbriae and long papillae along its surface, instead of large globular macro- and microtubercles and short fimbriae, as revised Pettibone (1975).
Parahermenia piotrowskiae sp. nov.
(Fig. 13)
http://zoobank.org/urn:lsid:zoobank.org:808A56BB-18
7E-4E60-97D9-DF4308793F2E
Diagnosis. Parahermenia with body papillate, elytra with globular macrotubercles and a single spine, and few filamentous papillae, margin fimbriate; neurochaetae with 2-3 accessory large teeth.
Description. Holotype (CAS 217917), complete, markedly bent ventrally, with abundant papillae along body; dorsum and vente white, first and last pairs of elytra whitish, first with 3 colorless macrotubercles, arranged in a triangle, last elytra with a single colorless globular macrotubercle; other elytra with globular brownish macrotubercles centrally, and long filamentous fimbriae. Second pair of elytra with 3 large globular central macrotubercles, arranged in a triangle (Fig. 13A), following elytra with smaller macrotubercles, arranged in an oblique row or a triangle (Fig. 13B). Some left median and posterior elytra detached; right elytron 6 and left parapodium of chaetiger 12 removed for observation (all kept in container). Body depressed, slightly tapered posteriorly, 6 mm long, 2 mm wide, 26 chaetigers.
Prostomium hexagonal, about as long as wide; median ceratophore 2 times as large and long as laterals, median ceratostyle lost; lateral ceratostyles tapered, subdistally swollen, 1/5 as long as palps, with reddish bands below and over swollen area, tips reddish. Palps papillate, with 2 longitudinal lateral reddish bands, middorsal area pale, with a subdistal reddish band, tips darker. Eyes black, of similar size, anterior eyes in wider prostomial area, posterior ones close to posterior margin.
Facial tubercle globular, small. Tentacular segment indistinct dorsally; second chaetiger without nuchal lappet, dorsum with transverse reddish bands, without tubercles; tentacular cirri resembling lateral antennae in pigmentation, slightly shorter than palps, chaetae not seen.
Ventral cirri of chaetiger 2 lost. Nephridial lobes not seen. Median elytra oval, overlapping laterally, not dorsally. Each with 2-3 large, central reddish or brownish globular macrotubercles, some with 4 (Fig. 13C), others with 2-3 macrotubercles, a single large falcate spine external to globular macrotubercles, and sparse papillae, most short (about as long as wide), a few 4-6 times longer than wide; fimbriae with cylindrical filaments, some distally swollen, 1/10-1/7 elytral length.
Parapodia biramous (Fig. 13D). Dorsal cirri resembling tentacular cirri, barely swollen subdistally, with brownish bands before and after a swollen dark area (Fig. 13D), ceratostyle with small papillae. Notochaetae thin, with a single series of fine denticles (Fig. 13D, inset below).
Neuropodia larger, prechaetal and postchaetal lobes blunt, papillate. Neurochaetae barely swollen subdistally, unidentate, with 2 large subdistal teeth (Fig. 13E).
Posterior region truncate; pygidium with anus terminal (Fig. 13F), anal cirri resembling dorsal cirri.
Taxonomic summary
Type material. Philippine Islands. Holotype (CAS 217917), Verde Island Passage Expedition 2016, Luzon Island, Batangas Province, Tingloy, Maricaban Island, Devil’s Point (13.65°N, 120.84°E), coral rubble, 40 m, 20 Apr. 2016, C. Piotrowski, coll.
Distribution. Only known from a subtidal locality (40 m) in Luzon Island, Philippines.
Etymology. This species is being named after M.Sc. Christina N. Piotrowski, Collection Manager of the Invertebrate Zoology collections in the California Academy of Sciences, in recognition of her support of our research activities, and because of her sampling efforts in many tropical localities of the world, especially because she collected the holotype.
Remarks
Parahermenia piotrowskiae sp. nov. resembles P. hermenioidea, the other only known species in the genus, because both have small bodies, and their elytra have long filamentous fimbriae. Their main differences are in the elytral ornamentation. In P. piotrowskiae elytra have globular, usually pigmented macrotubercles and a single spine, sparse filamentous papillae, and the fimbriae filaments are short, 1/10-1/7 as long as elytral length, whereas in P. hermenioidea the macrotubercles are markedly smaller, and there are no large spines, its filamentous papillae are long and abundant, and the fimbriae filaments are long, 1/2-1/3 of elytral length.
Key to species of Parahermenia gen. nov.
1 Median segments with elytral surface provided with large, reddish or brownish macrotubercles, a single large spine, papillation sparse; elytral fimbriae short (1/10-1/7 elytral length)
………………………………………………………………………………P. piotrowskiae sp. nov. Philippines
– Median segments with elytral surface provided with small, pale macrotubercles, without spines, papillation abundant; elytral fimbriae long (1/2-1/3 elytral length)
……………………………………………………………………………… P. hermenioidea (Amoureux, 1974) comb. nov. Madagascar.
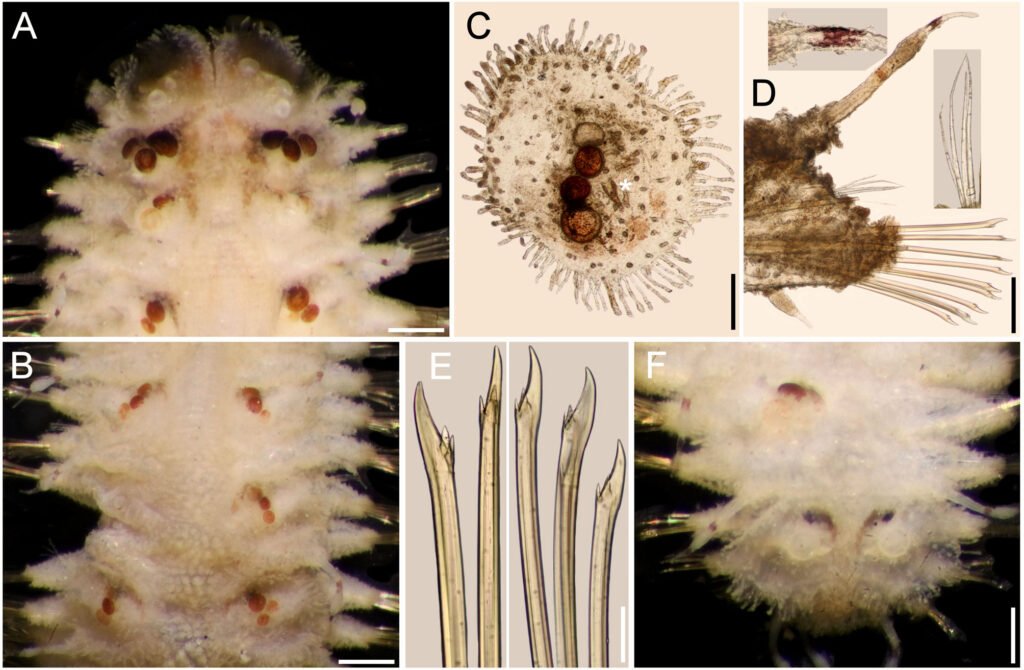
Discussion
The taxonomy of polynoid scaleworms has relied on morphological characters, such as the arrangement of cephalic appendages (subfamilies), and the features of the elytral and parapodial structures, including chaetae. Among Lepidonotinae, the traditional approach has been to use the number of pairs of elytra and other parapodial features for separating similar genera. In the group of lepidonotin genera provided with 12 pairs of elytra, the distinguishing features include the presence of some parapodial features (branchiae, pseudelytra) or types of chaetae. We have confirmed the distinguishing features of Hermenia such as the presence of reduced elytra along most body segments, and that neurochaetae have subdistal smooth regions, as opposed to most other genera having spinulose regions, and tips bi- or tridentate, opposed to being uni- or bidentate.
We have also refined the use of some distinguishing features for separating similar Hermenia species, and for newly describing other ones. They include the modifications of the nuchal lappet (distinctive, or indistinct), body integument (tuberculate, papillate, or both) and pigmentation, shape of elytra (mostly circular, some oval, wider than long), elytral size along body (similar to body end, or last pair larger than median ones), type of macrotubercles (most ovoid, a few basally swollen), and tips of neurochaetae (bi- or tridentate). Hermenia now includes 3 previously known species: H. verruculosa, described from the Caribbean Sea (type species), H. acantholepis, described from Philippines, and H. neoverruculoa, from Republic of Mauritius, and 4 new species: H. chuarae sp. nov. from Indonesia, H. mezianei sp. nov. from Vietnam, H. treadwelli sp. nov. from the Grand Caribbean, and H. wehei sp. nov. from the Arabian Sea and Indian Ocean.
Two other species, one already described, and another undescribed, resembled Hermenia species by having body papillate and neurochaetae subdistally smooth, but differed by having larger elytra overlapping laterally along body (restricted to a few anterior pairs in Hermenia), and elytra provided with long fimbria (short in Hermenia) and a few macrotubercles (very abundant in Hermenia). We proposed Parahermenia gen. nov. for these 2 species and separated them after the size of elytral fimbriae and number of macrotubercles. The new genus contains P. hermenioidea comb. nov. from Madagascar, and P. piotrowskiae sp. nov. from the Philippines. An interesting fact that no species of Hermenia or Parahermenia have been found along the Eastern Pacific or Western African coasts.
Acknowledgments
To Christina Piotrowski (CAS), Danny Eibye-Jacobsen and the late Mary Petersen (ZMUC), Leslie Harris (LACM), Marie-Louise Tritz and Ekin Tilic (SMF), Miranda Lowe and Emma Sherlock (BMNH), the late Nancy Voss and María Criales (UMML), Gisela Wegener and Angelika Brandt (ZMH), Fredrik Pleijel, Tarik Meziane, Laure Corbari and Stéphane Hourdez (MNHN), Gustav Paulay and Amanda Bemis (UF), kindly allowed us to study part of their collections. The then-known Consejo Nacional de Ciencia y Tecnología, México, funded the project Poliquetos del Gran Caribe (Conacyt 32529T), and provided a PhD scholarship to the first author (Conacyt 94497). The warm support by the late Kristian Fauchald and Len Hirsch, as well as Leslie Harris and David Ocker, Laure Corbari and Tarik Meziane, allowed us to make research visits to study their magnificent collections. Emilia González and Luis F. Carrera-Parra took care of the ECOSUR Reference Collection.
References
Amoureux, L. (1974). Annélides polychètes de Madagascar recueillies par G. Cherbonnier en 1960. Bulletin du Museum National d Histoire Naturelle, Paris, série 3, Zoologie, 217, 425–462.
Baird, W. (1865). Contributios towards a monograph of the species of Annelides belonging to the Aphroditacea, containing a list of the know species, and a description of some new species contained in the National Collection of the British Museum. Journal of the Linnean Society, Zoology, 8, 172–202. https://doi.org/10.1111/j.1096-3642.1865.tb02438.x
Bellan, G. (1964). Résultats scientifiques des campagnes de la Calypso dans l’Atlantique. Annélides polychetes. Annales de l’Institute Océanographique, 41, 301–314.
Ben-Eliahu, M. N. (1975). Polychaete cryptofauna from rims of similar intertidal vermetid reefs on the Mediterranean coast of Israel and in the Gulf of Elat: Sabellidae (Polychaeta Sedentaria). Israel Journal of Zoology, 24, 54–70. https://doi.org/10.1080/00212210.1975.10688411
Brown, R. W. (1954). Composition of scientific words: a manual of methods and a lexicon of materials for the practice of logotechnics. Baltimore: George W. King.
Chuar, C. H., Hadiyanto, H., & Lee, Y. L. (2021). Annotated checklist of polychaetes from deeper waters of the Sunda Strait and eastern Indian Ocean off southwest Java, Indonesia. Raffles Bulletin of Zoology, 36 (Supplement), 47–77. https://doi.org/10.26107/RBZ-2021-0030
Dawydoff, C. (1952). Contribution a l’étude des invertébrés de la faune marine benthique de l’Indochine. Bulletin Biologique de la France et de la Belgique, 37 (Supplement), 1–158.
Devaney, D. M. (1974). Shallow-water echinoderms from British Honduras, with a description of a new species of Ophiocoma (Ophiuroidea). Bulletin of Marine Science, 24, 122–164.
Ebbs, N. K. (1966). The coral-inhabiting polychaetes of the northern Florida reef tract, 1. Aphroditidae, Polynoidae, Amphinomidae, Eunicidae, and Lysaretidae. Bulletin of Marine Science, 16, 485–555.
Fauchald, K. (1977). The polychaeta worms: Definitions and keys to the orders, families and genera. Natural History Museum of Los Angeles County, Science Series, 28, 1–188.
Fauvel, P. (1922). Annélides polychètes de l’Archipel Hontman Abrolhos (Australie Occidentale) recueillies par M. le Prof. W.J. Dakin, F.L.S. Journal of the Linnean
Society,Zoology,34, 487–500. https://doi.org/10.1111/j.1096-3642.1922.tb01843.x
Fauvel, P. (1932a). Annelida Polychaeta of the Indian Museum, Calcutta. Memoirs of the Indian Museum, 12, 1–262.
Fauvel, P. (1932b). Mission Robert Ph. Dollfus en Égypte (Décembre 1927 – Mars 1929). Résumé analytique sur les polychètes. Bulletin de I institut d Égypte, 15, 131–144. https://doi.org/10.3406/bie.1932.3260
Fauvel, P. (1933). Mission Robert Ph. Dollfus en Égypte (Décembre 1927 – Mars 1929). Annélides polychètes. Bulletin de l’Institut d’Égypte, 21, 31–83.
Fauvel, P. (1935). Annélides polychètes de l’Annam. Memorie de lla Pontificia Accademia Romana dei Nuovi Lincei, série 3, 2, 279–354.
Fauvel, P. (1939). Annélides polychètes de l’Indochine recueillies par M. C. Dawydoff. Comment Pontificale Academie des Sciences, Civ Vaticana, 3, 243–368.
Fauvel, P. (1947). Faune de l’Empire Français, 8. Annélides polychètes de Nouvelle-Calédonie et des Iles Gambier. Paris: Office de la Recherche Scientifique Coloniale.
Fauvel, P. (1953a). The fauna of India including Pakiastan, Ceylon, Burma and Malaya: Annelida Polychaeta. Allhabad: Indian Press.
Fauvel, P. (1953b). Annélides polychétes de la Croisiére du Président Theodore Tissier aux Antilles. Bulletin de l’Institut Océanographique, Monaco, 1033, 1–23.
Grube, E. (1850). Die Familien der Anneliden. Archiv für Naturgeschichte, Berlin, 16, 249–364.
Grube, E. (1851). Uebersicht der Annelidengattungen und Arten zu ihrer vorläufigen Uterschidung. In Die Familien der Anneliden mit Angabe ihrer Gattungen und Arten. Ein systematischer Versuch (pp. 117–164). Berlin: Nicolai’schen Buchhandlung.
Grube, A. E. (1856). Annulata Örstediana Enumeratio Annulatorum, quae in itinere per Indiam occidentalem et Americam centralem annis 1845-1848 suscepto legit cl. A. S. Örsted, adjectis speciebus nonnullis a cl. H. Kröyer in itinere ad Americam meridionalem collectis. Havniae Part, 1, 44 – 62.
Grube, E. (1878). Annulata Semperiana. Memoires de l’Academie Imperiale des Sciences de St. Petersbourg, série 7, 25, 1–300.
Hanley, J. R., & Burke, M. (1991). Polychaeta Polynoidae: scaleworms of the Chesterfield Island and Fairway Reef, Coral Sea. In A. Crosnier (Ed.), Resultats des campagnes Musorstom 8 (pp. 9–82). Paris: Mémoires du Muséum National d’Histoire Naturelle.
Hartman, O. (1939). The polychaetous annelids collected on the Presidential Cruise of 1938. Smithsonian Miscellaneous Collections, 98, 1–22.
Herdman, W. A., & Hornell, J. (1903). Narrative with an outline of the investigation and details of the stations where observations were made. Report to the Government of Ceylon on the pearl oyster fisheries of the Gulf of Manaar, 1, 17–98.
Horst, R. (1917). Polychaeta Errantia of the Siboga-Expedition, 2. Aphroditidae and Chrysopetalidae. Siboga-Expeditie Monographie, Leyden, 24b, 1–140.
Horst, R. (1922). On some polychaetous annelids from Curaçao. Bijdragen tot de Dierkunde, 22, 193–201.
Imajima, M. (1997). Polychaetous annelids from Sagami Bay and Sagami Sea collected by the Emperor Showa of Japan and deposited at the Showa Memorial Institute, National Science Museum, Tokyo. Families Polynoidae and Acoetidae. National Science Museum, Tokyo Monographs, 13, 1–130.
Kinberg, J. G. H. (1856). Nya slägten och arter af Annelider. Animalia Annulata nova 1. Minus rite cognita recensuit. Öfversigt af Kongliga Vetenskaps-akademiens forhan-
dlingar, 12, 9–10, 381–388.
Monro, C. C. A. (1924). On the Polychaeta collected by H.M.S. ‘Alert’, 1881-1882. Families Polynoidae, Sigalionidae, and Eunicidae. Journal of the Linnean Society of London, Zoology, 36, 37–64. https://doi.org/10.1111/j.1096-3642.1924.tb02207.x
Monro, C. C. A. (1939). On some tropical polychaetes in the British Museum mostly collected by Dr. C. Crossland at Zanzibar, Tahiti, and the Marquesas, 1. Families Amphinomidae to Phyllodocidae. Annals and Magazine of Natural History, series 11, 4, 161–184.
O’Hara, T. D., Hugall, A. F., Cisternas, P. A., Boissin, E., Bribiesca-Contreras, G., Sellanes, J. et al. (2019). Phylogenomics, life history and morphological evolution of ophiocomid brittlestars. Molecular Phylogenetics and Evolution, 130, 67–80. https://doi.org/10.1016/j.ympev.2018.10.003
Pettibone, M. H. (1975). Review of the genus Hermenia, with a description of a new species (Polychaeta: Polynoidae: Lepidonotinae). Proceedings of the Biological Society of Washington, 88, 233–248.
Pettibone, M. H. (1977). Review of Halosydnopsis and related genera (Polychaeta: Polynoidae: Lepidonotinae). In D. J. Reish, & K. Fauchald (Ed.), Essays on polychaetous annelids in memory of Dr. Olga Hartman (pp. 39–62). The Allan Hancock Foundation, University of Southern California. Los Angeles.
Pettibone, M. H. (1993). Scaled polychaetes (Polynoidae) associated with ophiuroids and other invertebrates and review of species referred to Malmgrenia McIntosh and replaced by Malmgreniella Hartman, with descriptions of new taxa. Smithsonian Contributions to Zoology, 538, 1–92.
Pruvot, G. (1930). Annélides polychètes de Nouvelle-Calédonie recueillies par M. François. Archives de Zoologie Expérimentale et Générale, Paris, 70, 1–94.
Salazar-Silva, P., & Carrera-Parra, L. F. (2014). Revision of Lepidonopsis humilis (Augener, 1922) and description of L. barnichae sp nov (Annelida: Polychaeta: Polynoidae) based upon morphological and molecular characters. Zootaxa, 3790, 555–566. https://doi.org/10.11646/zootaxa.3790.4.4
Salazar-Vallejo, S. I. (1996). Lista de especies y bibliografía de poliquetos (Polychaeta) del Gran Caribe. Anales del Instituto de Biología UNAM, serie Zoología, 67, 11–50.
Salazar-Vallejo, S. I., & Eibye-Jacobsen, D. (2012). Annulata örstediana: Publication dates, composition and annotated taxonomic list, with some comments on Hemipodus (Polychaeta: Glyceridae). Revista de Biologia Tropical, 60, 1391–1402.
Seidler, H. J. (1923). Über neue und wenig bekannt Polychäten. Zoologischen. Anzeiger, Leipzig, 56, 254–264.
Seidler, H. J. (1924). Beitrage zur Kenntnis der Polynoiden, 1. Archiv für Naturgeschichte, 89A, 1–217.
Treadwell, A. L. (1911). Polychaetous annelids, from the Dry Tortugas, Florida. Bulletin of the American Museum of Natural History, 30, 1–12.
Wehe, T. (2006). Revision of the scale worms (Polychaeta: Aphroditoidea) occurring in the seas surrounding the Arabian Peninsula. Part 1. Polynoidae. Fauna Arabia, 22, 23–197.