Jesús García-Jiménez a, Olivia Ayala-Vásquez a, b, Javier Isaac de la Fuente a, b,
Roberto Garibay-Orijel c, Fortunato Garza-Ocañas d, Edgardo Ulises Esquivel-Naranjo e,
Felipe Manuel Ferrusca-Rico e, Fidel Landeros e, *
a Tecnológico Nacional de México, Instituto Tecnológico de Ciudad Victoria, Boulevard Emilio Portes Gil #1301, 87010 Ciudad Victoria, Tamaulipas, Mexico
b Colegio de Postgraduados, Microbiología, Edafología, Km 36.5 Carretera México-Texcoco, Montecillo, 56230 Texcoco, Estado de México, Mexico
c Universidad Nacional Autónoma de México, Instituto de Biología, Circuito exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Mexico City, Mexico
d Universidad Autónoma de Nuevo León, Campus Linares, Facultad de Ciencias Forestales, Carretera Nacional Km 145, 67700 Linares, Nuevo León, Mexico
e Universidad Autónoma de Querétaro, Facultad de Ciencias Naturales, Carretera a Chichimequillas s/n, 76140 Santiago de Querétaro, Querétaro, Mexico
*Corresponding author: landeros@uaq.mx (F. Landeros-Jaime)
Received: 1 May 2023; accepted: 25 March 2024
Abstract
Cyanoboletus abieticola is described as a new species to science. This species is characterized by the viscid pileus, cylindric to bacilliform basidiospores ([14.1] 16.2 ± 1.1 [17.5] × [5] 5.5 ± 0.3 [5.9] µm, Q [2.8] 3.0 ± 0.1 [3.2]), and its gregarious habit in mixed coniferous forests dominated by Abies religiosa or A. guatemalensis. Photographs, drawings, and the phylogenetic analysis of 3 genetic data sets (ITS, nucLSU, and RPB2) of the new species are presented.
Keywords: Boletales; Mycorrhizal fungi; “Pulveroboletus group”; Abies
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Cyanoboletus abieticola (Boletaceae, Basidiomycota), una especie nueva de México
Resumen
Cyanoboletus abieticola es descrita como una especie nueva para la ciencia. Esta especie se caracteriza por el píleo víscido, basidiosporas cilíndricas a baciliformes ([14.1] 16.2 ± 1.1 [17.5] × [5] 5.5 ± 0.3 [5.9] µm, Q [2.8] 3.0 ± 0.1 [3.2]) y su hábito gregario en bosques mixtos de coníferas dominados por Abies religiosa o A. guatemalensis. Se presentan fotografías, dibujos y el análisis filogenético de 3 conjuntos de datos genéticos (ITS, nucLSU y RPB2) de la nueva especie.
Palabras clave: Boletales; Hongos ectomicorrizógenos; “Grupo Pulveroboletus”; Abies
Introduction
The family Boletaceae comprises pileate-stipitate or sequestrate species, with poroid or lamellate hymenophore. Boletaceae species are an important component of ecosystems because most of them form ectomycorrhizal associations with a great diversity of angiosperms and gymnosperms (Binder & Hibbet, 2006; Ortíz-Santana et al., 2007). They are common in different vegetation types, such as deciduous, conifer, and mixed forest; mediterranean shrublands, tropical and montane cloud forests (Bessette et al., 2010; Gelardi, 2020; Leonardi et al., 2020; Wu et al., 2016). In addition to their ecological importance, many species are valued edibles in several regions of the world (Garibay-Orijel et al., 2009; Gelardi, 2020).
Wu et al. (2014) recognized 7 major clades at the subfamily level and 59 genus-level clades, placing Boletus pulverulentus within the “Pulveroboletus group”, which also includes the genera Butyriboletus D. Arora & J.L. Frank, Cacaoporus Raspé & Vadthanarat, Caloboletus Vizzini, Crocinoboletus N.K. Zeng, Zhu L. Yang & G. Wu, Gymnogaster J.W. Cribb, Lanmaoa G. Wu & Zhu L. Yang, Pulveroboletus Murrill, Rubroboletus Kuan, Rugiboletus G. Wu & Zhu L. Yang, Suillellus Murrill, and Sutorius Halling, Nuhn & N.A. Fechner. Members of the “Pulveroboletus group” share certain common characteristics such as poroid hymenium, usually yellowish, reddish or brown hymenophore, Boletus-type hymenophoral trama (Snell & Dick, 1958), and smooth spores.
Gelardi et al. (2014) proposed the new genus Cyanoboletus, within the “Pulveroboletus group”, for those species characterized mainly by their strong blue reaction of basidiomata and context to cutting. They included 3 species in this genus: C. pulverulentus (Opat.) Gelardi, Vizzini & Simonini, C. sinopulverulentus (Gelardi & Vizzini) Gelardi, Vizzini & Simonini, and C. rainisiae (Bessette & O.K. Mill.) Gelardi, Vizzini & Simonini. The latter one was transferred to the genus Xerocomellus, but without type studies, and is currently known as X. rainisiae (Bessette & O.K. Mill.) N. Siegel, C.F. Schwarz & J.L. Frank (see discussion in Frank et al. [2020]).
Later, 4 more species were transferred to this genus: C. cyaneitinctus (Murrill) A. Farid, A.R. Franck & J.A. Bolin (Farid et al., 2021), C. instabilis (W. F. Chiu) G. Wu & Zhu L. Yang (Wu et al., 2016), C. poikilochromus (Pöder, Cetto & Zuccherelli) M. Carbone, D. Puddu & P. Alvarado (Carbone et al., 2023), and C. flavosanguineus (Lavorato & Simonini) Pierotti (Pierotti, 2015). However, the latter species was placed in the genus Neoboletus in 2021: N. flavosanguineus (Lavorato & Simonini) Biketova, Wasser, Simonini & Gelardi (Biketova et al., 2021).
Additionally, 5 Cyanoboletus species have been recently described: C. brunneoruber G. Wu & Zhu L. Yang from China (Wu et al., 2016), C. hymenoglutinosus D. Chakr., K. Das, A. Baghela, S.K. Singh & Dentinger nom. inval. from India (Li et al., 2016), C. bessettei A.R. Bessette, L.V. Kudzma, & A. Farid from the USA (Farid et al., 2021), C. macroporus Sarwar, Naseer & Khalid from Pakistan (Sarwar et al., 2021), and C. mediterraneensis Biketova, A. Rinaldi & Simonini from Israel and Italy (Biketova et al., 2016, 2022). Therefore, 10 species are currently known for the genus. These species are distributed mainly in Asia, Europe and North America and associated with Abies, Arbutus, Buxus, Carya, Castanopsis, Cistus, Crataegus, Cryptomeria, Erica, Juniperus, Lithocarpus, Ostrya, Pinus, Pistacea,and Quercus trees, as well as with Halimium shrub (Biketova et al., 2022; Farid et al., 2021; García et al., 1998; Li et al., 2016; Wu et al., 2016).
This paper describes Cyanoboletus abieticola as a new species from Mexico associated with mixed conifer forests dominated by Abies religiosa (Kunth) Schltdl. & Cham. and A. guatemalensis Rehder.
Materials and methods
Collections were carried out in central and southern Mexico (Fig. 1), in mixed coniferous forests, during the rainy season from June to October (2000-2017). Morphological characteristics were described according to Largent (1977) and Lodge et al. (2004). Chemical reactions with KOH 5% and Melzer reagent were tested on pileus, hymenophore, and stipe surface. Photographs of basidiomata were taken on site, as well as data regarding plant species. The colors for the taxonomic description were based on Kornerup and Wanscher (1978). A light microscope (Carl Zeiss GmbH 37081, Germany) was used to observe the microscopic characteristics. Only structures from mature basidiomes were measured. Twenty basidiospores, basidia, pleurocystidia, cheilocystidia and caulocystidia were measured by specimen. If the specimen consisted of several basidiomes, only the structures of 2 of them were measured. The mean of each variable of every collection was computed. Dimensions are given according to the following format: (lower mean) mean of means ± standard deviation (upper mean), Q = (lower mean) mean of means ± standard deviation (upper mean).
Vouchers were deposited in the “Herbario Nacional de México” (MEXU), in the José Castillo Tovar herbarium (ITCV), and in the mycological herbarium of the Universidad Autónoma de Querétaro (MUAQ). Additional materials were obtained in loan from the following herbaria: Escuela Nacional de Ciencias Biológicas-Instituto Politécnico Nacional herbaria (ENCB-IPN), Instituto de Biología Universidad de Guadalajara (IBUG), Instituto Nacional de Ecología (INECOL) and Universidad Autónoma de Nuevo León (UNL). All acronyms of herbaria and mycological collections follow Index Herbariorum (Thiers, 2024).
Samples of dehydrated basidiomata were used for DNA extraction. The DNA was extracted using the DNeasy Power-Soil kit (QIAGEN). Cell lysis was performed by grinding in a mortar with liquid nitrogen. Three nuclear loci (ITS, LSU and RPB2) were amplified with Platinum Taq DNA Polymerase (Invitrogen-Thermo Fisher Scientific) or Taq & Load PCR Mastermix (MP Biomedicals) in a thermal cycler (BIO-RAD). The PCR parameters were as follows: 95 ºC initial denaturation 4 min; 35 cycles of denaturation at 94 ºC for 1 min, annealing at 54 ºC for 1 min, extension at 72 ºC for 1 min, and a final extension step at 72 ºC for 10 min. The primers ITS1/ITS4 (White et al., 1990) were used for the ITS region; LR0R/LR5 (Vilgalys & Hester, 1990) for nucLSU; and RPB2-B-F2/RPB2-B-R (Wu et al., 2014) for the RPB2 gene. The PCR products were examined by 1% agarose gel electrophoresis, the gel was stained with GelRed (Biotium) and observed under an UVP Multidoc-It transilluminator (Analytikjena). Successful PCR products were cleaned with diluted 1:1 ddH2O: ExoSAP-IT (Thermo Fisher Scientific) and incubated at 37 ºC for 45 min and 80 ºC for 15 min. Sanger sequencing of clean PCR products was performed in the “Laboratorio de secuenciación genómica de la biodiversidad y la salud” at Instituto de Biología, Universidad Nacional Autónoma de México. Samples were sequenced in both directions with PCR primers using BigDye Terminator v3.1 (Thermo Fisher Scientific). Sequences were edited in Chromas Pro Vers. 1.41 (Technelysium Pty, Ltd, Tewantin, Qld, Australia).
For phylogenetic analyses we used Cyanoboletus sequences from the ITS, nucLSU and RPB2 regions listed in the Table 1. A total of 32 concatenated sequences were used (Supplementary material 1). Two Lanmaoa species were chosen as an outgroup. The sequences of each locus were aligned separately using MacClade 4.0 (Maddison & Maddison, 2000) and concatenated in Mesquite 3.40 (Maddison & Maddison, 2018). The alignments were manually edited to eliminate ambiguous regions.
Table 1
List of DNA sequences used for Cyanoboletus phylogenetic analyses.
| Species | Code | Country | GenBank | Reference | ||
| ITS | nucLSU | RPB2 | ||||
| Lanmaoa sublurida | USF 288426 | USA | MW675740 | MW662575 | MW737499 | Farid et al., 2021 |
| L. sublurida | USF 300104 | USA | MW675736 | MW662572 | MW737498 | Farid et al., 2021 |
| Cyanoboletus abieticola | MUAQ13 Paratype | Mexico | MW750332 | MW750369 | – | This study |
| C. abieticola | ITCV-1010 Paratype | Mexico | – | MW750367 | – | This study |
| C. abieticola | MEXU-30111 Paratype | Mexico | MW209739 | MW750366 | PP108649 | This study |
| C. abieticola | MEXU-30106 Paratype | Mexico | MW209740 | MW750365 | – | This study |
| C. abieticola | MEXU-30109 Holotype | Mexico | MW209738 | MW750368 | PP108650 | This study |
| Table 1. Continued | ||||||
| Species | Code | Country | GenBank | Reference | ||
| ITS | nucLSU | RPB2 | ||||
| C. abieticola | MEXU-26275 Paratype | Mexico | KC152077 | – | – | This study |
| C. abieticola | MEXU-26276 Paratype | Mexico | KC152076 | – | – | This study |
| C. abieticola | MEXU-26278 Paratype | Mexico | KC152075 | – | – | This study |
| C. bessettei | USF 301500 (A) Holotype | USA | MW675737 | MW662571 | MW737457 | Farid et al., 2021 |
| C. bessettei | USF 301500 (B) Holotype | USA | MW675738 | – | MW737458 | Farid et al., 2021 |
| C. brunneoruber | HKAS63504 | China | – | KF112368 | KF112702 | Wu et al., 2014 |
| C. brunneoruber | HKAS80579 1 | China | – | KT990568 | KT990401 | Wu et al., 2016 |
| C. brunneoruber | HKAS80579 2 | China | – | KT990569 | KT990402 | Wu et al., 2016 |
| C. cyaneitinctus | USF 288424 | USA | MW675739 | MW662574 | MW737461 | Farid et al., 2021 |
| C. cyaneitinctus | USF 301499 Epitype | USA | MW675744 | MW662579 | MW737503 | Farid et al., 2021 |
| C. cyaneitinctus | JAB184 | USA | MW675731 | MW662584 | MW737467 | Farid et al., 2021 |
| C. cyaneitinctus | JAB324 | USA | MW675732 | MW662586 | MW737469 | Farid et al., 2021 |
| C. cyaneitinctus | JAB325 | USA | MW675733 | – | MW737470 | Farid et al., 2021 |
| C. hymenoglutinosus | AB2016 | India | KT907355 | KT860060 | – | Li et al., 2016 |
| C. instabilis | FHMU1839 | China | MG030473 | MG030466 | – | Chai et al., 2018 |
| C. instabilis | HKAS59554 | China | – | KF112412 | KF112698 | Wu et al., 2014 |
| C. macroporus | DC21-02 | India | OQ860238 | OQ860239 | ON364552 | Das et al., 2023 |
| C. macroporus | DC21-04 | India | OQ860240 | OQ860241 | OQ876894 | Das et al., 2023 |
| C. mediterraneensis | K-M000265123 Holotype | Israel | – | OM801212 | – | Biketova et al., 2023 |
| C. mediterraneensis | K-M000265124 Paratype | Israel | OM801199 | – | – | Biketova et al., 2023 |
| C. mediterraneensis | TUR-A 209199 | Italy | MZ265183 | MZ265198 | MZ277228 | Carbone et al., unpublished |
| C. paurianus | KD22-008 | India | – | OQ859920 | OQ914389 | Das et al., 2023 |
| C. paurianus | KD22-009 Holotype | India | – | OQ859919 | OQ914388 | Das et al., 2023 |
| C. poikilochromus | GS10070 | Italy | KT157051 | KT157060 | KT157068 | Gelardi et al., 2015 |
| C. poikilochromus | GS11008 | Italy | KT157050 | KT157059 | KT157067 | Gelardi et al., 2015 |
| C. poikilochromus | TO HG 100091987 Epitype | Italy | KT157047 | KT157056 | – | Gelardi et al., 2015 |
| C. pulverulentus | MG126a | Italy | KT157053 | KT157062 | – | Gelardi et al., 2015 |
| C. pulverulentus | MG 456a | Portugal | KT157054 | KT157063 | – | Gelardi et al., 2015 |
| C. pulverulentus | MG 628a | Italy | KT157055 | KT157064 | KY157069 | Gelardi et al., 2015 |
| C. sinopulverulentus | HKAS59609 | China | – | KF112366 | KF112700 | Wu et al., 2014 |
| C. sinopulverulentus | HMAS266894 | China | KC579402 | – | – | Gelardi et al., 2015 |
| Cyanoboletus. sp. | HKAS76850 | China | – | KF112343 | KF112697 | Wu et al., 2014 |
Phylogenetic analyses were partitioned for both Maximum Likelihood (ML) and Bayesian Inference (BI) analyses. Best evolutionary models were selected with jModelTest 2.1.10 (Darriba et al., 2012), using the Akaike information criteria. Table 2 summarizes the evolutionary model of each region. The ML analysis was performed in RAxML 7.2.6 (Stamatakis, 2006), with 1,000 bootstrap replicates. The BI analysis was performed in MrBayes 3.2.5 (Ronquist & Huelsenbeck, 2003) with 5,000,000 generations, 4 Monte Carlo chains and sampling every 100 trees, chain convergence was determined using Tracer 1.7.2 (Rambaut et al., 2018), we discarded the first 25% of trees as burning. ML bootstrap support (BS) over 50% and Bayesian posterior probability (PP) values exceeding 0.90 are reported in the resulting tree.
Table 2
Nucleotide substitutions models by each partition obtained from jModelTest with the corrected Akaike information criterion.
| Gene/Partition | Length | Evolutifon model | Nst-rates |
| nucLSU | 747 pb | TIM1+G | 6 – Gamma |
| ITS | 603 pb | TPM3ef+G | 6 – Gamma |
| RPB2 | 688 pb | TIM3ef+G | 6 – Gamma |
Results
The phylogenetic analyses (Fig. 1) based on the ITS, nucLSU and RPB2 concatenated dataset using ML analyses and BI showed that all Cyanoboletus species cluster in a monophyletic clade with strong support (PP 1 / BS 100). Trees obtained from separate ITS and RPB2 analyses are presented in Supplementary material 2. Sequences of all C. abieticola sp. nov. samples form a strongly supported clade (PP 1/BS 99) within the genus Cyanoboletus. Consequently, based on morphological, phylogenetic and ecological data, we describe it as a new species, which is also the first species of the genus Cyanoboletus reported from Mexico.
To determine whether the ITS or RPB2 region is more informative as a barcode, in Table 3 we show the percentages of nucleotide similarity between species. The RPB2 region commonly has more variation and generates fewer ambiguous regions than the ITS. Even while the ITS region has been considered as the fungal genetic barcode, the RPB2 region has higher resolution for species delineation in Cyanoboletus.
Description
Cyanoboletus abieticola J. García, Ayala-Vásquez & Landeros,sp. nov.
Diagnosis. Pileus 13-50 mm in diameter, viscid, widely convex, convex to plane convex, brown, yellow, pale brown, reddish-brown, cinnamon. Stipe 40-75 × 7-8 mm, viscid, cylindrical, yellow when young, middle section and apex yellow when mature, basal area red brown to red-vinaceous, and basidiospores (14.1) 16.2 ± 1.1 (17.5) × (5) 5.5 ± 0.3 (5.9) µm. It grows on the ground of neotropical Abies forests.
Description. Macroscopic characters (Fig. 2). Pileus 13-50 mm diameter, widely convex, convex to plane convex, brown yellow (4B6), pale brown (6B5), reddish-brown, cinnamon, furfuraceous, very viscid when young, incurved margin, sterile. Hymenophore attached, pores 0.3-0.7 mm in diameter, pale yellow (2A8-2A4), yellow (3A8) to yellow-olive (30B8-30B7) it stains dark blue (22F8) when touched, with some brown tones, young specimens exude a somewhat acidic astringent-flavored yellow liquid from hymenophore; tubes 2-6 mm diameter, concolor to pores, immediately changing to dark blue (25F8) when cut. Context white, 5 mm thick, dark blue (22F8) when cut; stipe context pale yellow (3A6) base red-vinaceous (10F8), turning blue (25F8) when cut. Stipe 40-75 × 7-8 mm, cylindrical, yellow when young, middle section and apex yellow when mature, basal area red brown (9C8-9C5) to red-vinaceous (10F8), surface pruinose to furfuraceous, immediately turning dark blue (22F8) when touched. Mycelium white.
Chemical reactions: pileus surface and context turning dark brown (6F8) with KOH 5%, hymenophore turning brown (5F3) with KOH 5%.
Microscopic characters (Fig. 3). Basidiospores 14.1-17.5 × 5-5.9 µm, mean values 16.2 ± 1.1 × 5.5 ± 0.3 µm, Q 2.8-3.2, means values 3.0 ± 0.1, cylindric to bacilliform, yellow in KOH, inamyloid with Melzer’s reagent, with visible suprahilar depression (Fig. 3A). Basidia 27.3-37.6 × 9.5-10.7 µm, mean values 33.3 ± 4.0 × 10.3 ± 0.5 µm, clavate, hyaline in KOH, tetrasporic (Fig. 3B). Hymenophoral trama divergent (Boletus-type), with a medium and lateral stratum of cylindrical hyphae, hyaline to yellowish brown in KOH, inamyloid with Melzer’s reagent, with gelatinized wall. Pleurocystidia 52.2-71.2 × 10.6-13.9 µm, mean values 63.2 ± 7.5 × 12.4 ± 1.4 µm, arise from subhymenium, mucronate, clavate, fusoid-ventricose, hyaline to brown in KOH (Fig. 3C), with reddish brown incrustations on Melzer. Cheilocystidia 39.6-58.4 × 8.9-11 µm, mean values 47.7 ± 7.5 × 9.9 ± 0.8 µm, fusoid-ventricose, mucronate, clavate, reddish to brown on KOH, with reddish brown incrustations (dextrinoid) in Melzer’s reagent, thick-walled (Fig. 3D). Pileipellis formed by an ixotrichoderm 250-300 µm thick, with terminal cells 34.8-45.4 × 4-5.6 µm, mean values 38.4 ± 4.8 × 4.7 ± 0.6 µm, cylindrical, yellow-reddish brown in KOH, reddish brown (dextrinoid) with Melzer’s reagent, some with thick wall, sometimes gelatinized (Fig. 3E). Stipitipellis 100-120 µm thick, ixocutis, hyphae subparallel to loosely intermingled, formed of caulocystidia 34-47 × 9.6-11.4 µm, mean values 40.6 ± 5.2 × 10.3 ± 0.7 µm, in clusters, fusoid-mucronate, clavate, some ventricose, arise from the middle or surface, hyaline to brown in KOH, with reddish brown incrustations (dextrinoid) in Melzer’s reagent.
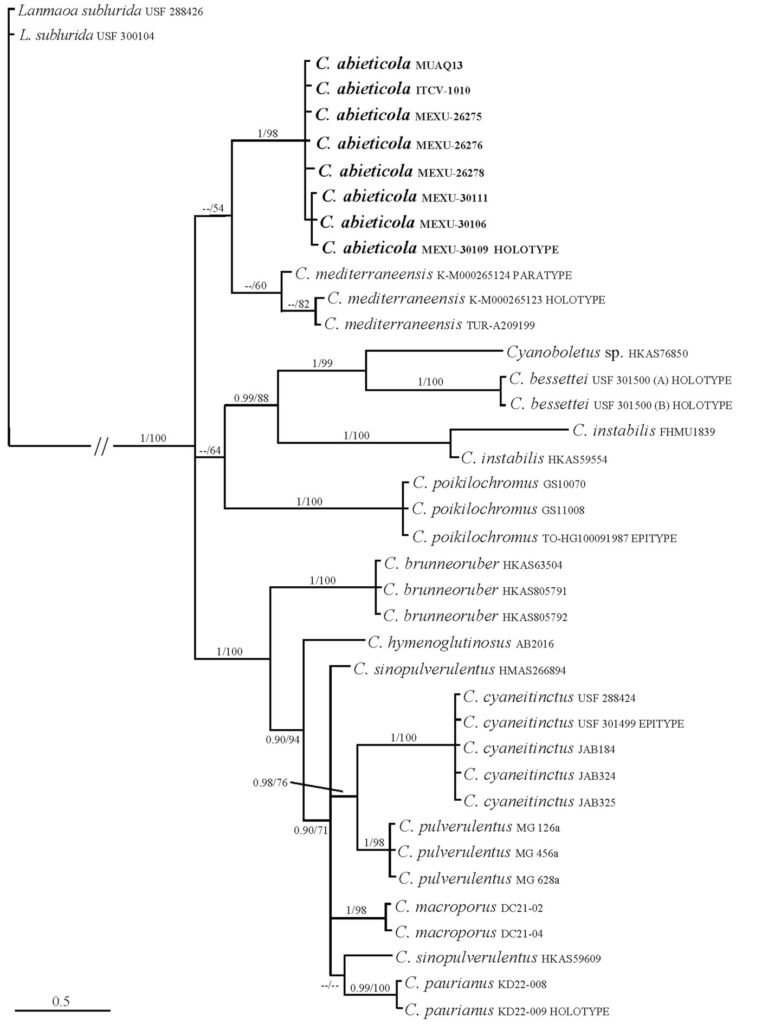
Figure 1. Bayesian tree using the concatenated alignment (LSU, ITS and RPB2). The values in the phylogram branches represent the maximum likelihood bootstrap (MLB)/Bayesian posterior probabilities (BPP). Only MLB 50 ≥ and 90 BPP ≥ are displayed. Cyanoboletus abieticola is in bold.
Taxonomic summary
Holotype: Mexico, Oaxaca, Santa Catarina Ixtepeji, La Cumbre, Abies guatemalensis, 17º11’ N, 96º38’ W, 2,902 m asl, 4 November 2017, Ayala-Vásquez (MEXU-30109).
Isotype: ITCV-1141
Mycobank: MB 838523.
Etymology: the name of the species is due to its association with Abies forests.
Habitat: scattered or solitary in mixed neotropical conifer forests dominated by Abies religiosa and A. guatemalensis.
Table 3
Percent nucleotide similarity between Cyanoboletus species based on their sequences, ITS/RPB2 DNA sequences. Above ITS and below RPB2 percentage ranges (WD: without data).
| C. abieticola | C. bessettei | C. brunneoruber | C. cyaneitinctus | C. hymenoglutinosusus | C. instabilis | C. macroporus | C. mediterraneensis | C. paurianus | C. poikilochromus | C. pulverulentus | C. sinopulverulentus | |
| C. abieticola | 0.0-0.5 0.0 | |||||||||||
| C. bessettei | 5.6-6.2 8.7 | 0.0 0.0 | ||||||||||
| C. brunneoruber | WD 5.9 | WD 8.7 | WD 0.0 | |||||||||
| C. cyaneitinctus | 6.7-7.2 7.0 | 8.1 10.2 | WD 5.9 | 0.0 0.0 | ||||||||
| C. hymenoglutinosus | 3.6-3.9 WD | 4.6 WD | WD WD | 5.7 WD | WD WD | |||||||
| C. instabilis | 5.7-7.0 7.5 | 5.7 8.3 | WD 7.8 | 6.3 8.7 | 6.3 WD | WD WD | ||||||
| C. macroporus | 4.4-4.6 6.0 | 5.4 8.8 | WD 5.3 | 4.9 3.6 | 1.8 WD | 7.0 7.9 | 0.0 0.0 | |||||
| C. mediterraneensis | 2.9-3.9 2.7 | 2.9-3.4 7.0 | WD 5.4 | 2.0-5.9 6.0 | 2.3 WD | 5.1 5.8 | 3.1-3.2 5.4 | 0.0-0.3 WD | ||||
| C. paurianus | WD 6.2 | WD 9.2 | WD 4.6 | WD 3.6 | WD WD | WD 8.0 | WD 2.7 | WD 4.9 | WD 0.0 | |||
| C. poikilochromus | 4.2-5.9 6.9-7.1 | 3.9 8.5-8.7 | WD 7.1-7.4 | 5.9-7.6 7.7-7.8 | 2.0-3.4 WD | 7.0-7.1 7.8-8.0 | 3.3-4.6 7.5 | 1.7-3.0 6.3-6.7 | WD 7.1-7.2 | 0.0 0.0-0.5 | ||
| C. pulverulentus | 3.6-3.9 6.5 | 4.9-5.2 9.1 | WD 5.2 | 4.6 2.2 | 2.1-2.3 WD | 6.3 8.4 | 1.8-2.1 2.8 | 2.3-2.8 5.1 | WD 2.8 | 3.6-4.9 7.8 | 0.0-0.3 WD | |
| C. sinopulverulentus | 3.8-4.1 6.1 | 5.2 9.3 | WD 4.6 | 4.0 3.9 | 2.8 WD | 6.3 7.4 | 1.0 2.7 | 2.3-3.1 4.7 | WD 2.1 | 3.1-4.7 7.0-7.1 | 1.3-1.6 3.3 | WD WD |
Additional material studied. Mexico, Hidalgo: El Chico National Park, Abies religiosa, 13 August 1988, J. García (ITCV-5874), 13 August 1988, J. García (ITCV-5878), 23 August 2018, J. García (ITCV-21996); Jalisco: Road to Tamazula, 15 km to Mazamitla, between Guayabos and Cabañas, A. religiosa, 24 August 1974, G. Guzmán (ENCB-11869); Road from San Sebastián del Oeste to Santa Ana, A. religiosa, 29 August 1994, L. Guzmán-Dávalos (IBUG-5349); Nevado de Colima, A. religiosa, 13 October 1984, A. Tamayo and R. González (IBUG); 11 August 1990, J. García (ITCV-6633); Estado de México: Road to Chalma, Lagunas de Zempoala National Park, A. religiosa, 1 August 1982, R. E. Chio (ENCB-368, ITCV-368), 23 September 1985, E. Perez-Silva (MEXU-19733), 17 July 1985, García (ITCV-4784); Nevado de Toluca National Park, near Ranchería La Puerta, A. religiosa, 21 August 1983, L. Colón 208-a (ENCB); Naucalpan-Toluca highway, road to Villa Alpina, La Glorieta, A. religiosa, without date, A. González-Velázquez (ENCB-965); Mpio. Amanalco, Corral de Piedras, A. religiosa, without date, A. González-Velázquez (ENCB-1454); Zone E of San Rafael Atlixco, Escualango, A. religiosa, 7 October 1983, A. Hernández (ENCB-145); La Marquesa, A. religiosa, 7 July 1963, Gispert-Imaz (MEXU-9873), 12 August 1962, G. Guzman (ENCB-3198); Ciudad de México: Former Mexico-Cuernavaca highway 3 km S. of Parres, A. religiosa, 17 July 1982, S. Chacón (ENCB-183); Michoacán: Morelia-Cd Road. Hidalgo, Sierra de Mil Cumbres, km 45, A. religiosa, 21 July 1983, J. García (UNL-3665, ITCV-3665), 15 July 1982, J. García (UNL-1998, ITCV-1998); Morelos: Road to Chalma, 5 km Huitzilac, A. religiosa, 17 July 1982, S. Chacón (ENCB-234); Oaxaca: Santa Catarina Ixtepeji, La Cumbre, 17°11’ N, 96°38’ W, A. guatemalensis, 18 July 2017, Ayala-Vásquez (ITCV-846), 20 August 2017, Ayala-Vásquez (ITCV-1002), 21 August 2017, Ayala-Vásquez and Victores-Aguirre, (ITCV-1010); 8 October 2017, Ayala-Vásquez, (MEXU-30106, ITCV-1129), Ayala-Vásquez (MEXU-30111, ITCV-1128); 4 November 2017, Ayala-Vásquez (ITCV-1136); Ayala-Vásquez (MEXU-30109, ITCV-1141), Ayala-Vásquez, (ITCV-1142); Querétaro: Mpio. Cerro El Zamorano, A. religiosa, 16 September 1995, J. García (ITCV-9560), 23 September 2017, Ferrusca 390 (MUAQ13), 19 october 2019, Ferrusca 462 (MUAQ14), Ferrusca 467 (MUAQ15), 16 september 2022, Landeros 3770 (MUAQ16); Tlaxcala: road from Tlaxco to Chignahuapan, Cerro de Teapa, El Conejo, A. religiosa, 26 June 1979, J. García (UNL-496, ITCV-496); Huamantla, road to La Malinche hilltop, A. religiosa, 29 August 2010, T. Bruns (MEXU-26275) (MEXU-26276) (MEXU-26278); Veracruz: Xico, Los Gallos, 1.5 km to N. from Ingenio El Rosario, Zona del Cofre de Perote, A. religiosa, 27 October 1983, Villarreal 1024 (INECOL-1068), 31 July 1990, J. García (ITCV-6459).

Figure 2. Cyanoboletus abieticola morphology. A) Basidiomata (holotype), B) context, C) basidiomata at different stages. Bar = 10 mm.

Figure 3. Microscopic characteristics of Cyanoboletus abieticola. A) Basidiospores, B) basidia, C) cheilocystidia, D) pleurocystidia, E) stipitipellis, F) pileipellis. Bar = 10 µm.
Remarks
Cyanoboletus abieticola is a new species with a strong phylogenetic support (PP1 / BS 99) and distinctive taxonomic characters. This species is characterized by a small pileus 13-50 mm diameter, very viscid, pale brown, yellowish-brown, brown, red to cinnamon; pileus sterile margin somewhat involute when young; hymenophore distills a liquid with sweaty acidic flavor when young, pores and tubes yellow; stipe yellow cylindrical in the middle and apex, basal area red brown to red-vinaceous, when touched immediately turns to dark blue. It is distributed in mixed coniferous forests in central and southern Mexico at altitudes ranging from 2,700 to 3,000 m asl (Fig. 4).

Figure 4. Distribution of Cyanoboletus abieticola in Mexico.
Previously, only 2 species of Cyanoboletus (C. bessettei and C. cyaneitinctus) have been recorded from North America (Farid et al., 2021). Cyanoboletus bessettei and C. cyaneitinctus have similar small basidiomata as C. abieticola, nevertheless, both species are associated with oak forests in the United States, in contrast, C. abieticola is distributed from central to southern Mexico in mixed conifer forests with a strong association with Abies. Morphological and microscopical comparisons of the American species of Cyanoboletus are shown in the Table 4. They are similar by morphology, however, C. bessettei has blue-green then reddish-brown staining in its stipe when handled, while C. abieticola and C. cyaneitinctus are bluing, also their basidiospores are bigger than those in C. bessettei (9-11 ´ 3.5-5 mm). Cyanoboletus cyaneitinctus and C. abieticola are very similar by morphology and microscopical characters, however they are not phylogenetically close, and the first one has shorter basidiospores in length (11.5-15 mm) and associates with Carya and Quercus.
Table 4
North American Cyanoboletus species morphology comparison.
| Characteristic | C. bessettei | C. cyaneitinctus | C. abieticola |
| Pileus color | Buffy brown when young, darker brownish at maturity | Bister, umber, mahogany and dark brown | Brown yellow, pale brown, reddish brown, cinnamon |
| Pileus size | 2.7-8 cm wide | 3-8 cm wide | 1.3-5 cm wide |
| Staining stipe when handled | Blue-green then reddish-brown | Bluing instantly and strongly | Bluing instantly and strongly |
| Stipe size | 2.5-4 × 1-2 cm | 3-6 × 0.5-2 cm | 4-7.5 × 0.7-0.8 cm |
| Basidia | (2)4-spored | 4-spored | 4-spored |
| Basidiospores | (8-)9-11(-12) × 3.5-5 mm | (11)11.5-15(16) × 4-6 mm | [(14.1) 16.2 ± 1.1 (17.5) × (5) 5.5 ± 0.3 (5.9) µm |
| Host plants | Under Quercus and Pinus | Under Carya and Quercus | Under Abies spp. |
Most Cyanoboletus species are morphologically similar, so to separate them, a combination of microscopic, geographic and molecular characteristics is necessary. With the description of this new species, the genus Cyanoboletus reaches its southernmost distribution in America, developing in forests of central and southern Mexico forests. Its occurrence in Abies forests is also highlighted, since in North America the genus has been recorded in mixed Pinus–Quercus forests (Farid et al., 2021).
Acknowledgements
We thank Instituto Tecnológico de Ciudad Victoria, Universidad de Quintana Roo, Universidad Nacional Autónoma de México and Universidad Autónoma de Nuevo León for supporting this research. The DNA sequences produced at IB-UNAM were financed by project Conacyt 239266 assigned to RGO. AVO thanks Conahcyt for the postdoctoral funding 3129307. The last author wants to thank the Universidad Autónoma de Querétaro for the project FNB-2022-06.
References
Bessette, A., Roody, W. C., & Bessette, A. R. (2010). Boletes of Eastern North America: a color guide to the fleshy pored mushrooms. China: Syracuse University Press.
Biketova, A. Y., Kosakyan, A., Wasser S. P., & Nevo, E. (2016). New, noteworthy, and rare species of the genus Boletus in Israel. Plant Biosystems, 150, 876–886. https://doi.org/10.1080/11263504.2014.990537
Biketova, A. Y., Wasser, S. P., Simonini, G., & Gelardi, M. (2021). Nomenclatural novelties: Neoboletus flavosanguineus (Lavorato & Simonini) Biketova, Wasser, Simonini & Gelardi, comb.nov. Index Fungorum, 505, 1.
Biketova A. Y., Rinaldi A. C., & Simonini, G. (2022). Nomenclatural novelties: Cyanoboletus mediterraneensis Biketova, A. Rinaldi & Simonini, sp. nov. Index Fungorum, 516, 1.
Binder, M., & Hibbett, D. S. (2006). Molecular systematics and biological diversification of Boletales. Mycologia, 98, 971–983. https://doi.org/10.1080/15572536.2006.11832
626
Carbone, M., Puddu, D., & Alvarado, P. (2023). Nomenclatural novelties: Cyanoboletus poikilochromus (Pöder, Cetto & Zuccherelli) M. Carbone, D. Puddu & P. Alvarado, comb. nov. Index Fungorum, 534, 1.
Darriba, D., Taboada, G. L., Doallo, R., & Posada, R. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772.
Farid, A., Bessette, A. E., Bessette, A. R., Bolin, J. A., Kudzma, L. V. Franck, A. R. et al. (2021). Investigations in the boletes (Boletaceae) of southeastern USA: four novel species and three novel combinations. Mycosphere, 12, 1038–1076. https://doi.org/10.5943/mycosphere/12/1/12
Frank, J. L., Siegel, N., Schwarz, C. F., Araki, B., & Vellinga, E. C. (2020). Xerocomellus (Boletaceae) in western North America. Fungal Systematics and Evolution, 5, 265–288. http://dx.doi.org/10.3114/fuse.2020.06.13
García, J., Pedraza, D., Silva, C. I., Andrade, R. L., & Castillo, J. (1998). Hongos del estado de Querétaro. Querétaro: Universidad Autónoma de Querétaro.
Garibay-Orijel, R., Martínez-Ramos, M., & Cifuentes, J. (2009). Disponibilidad de esporomas de hongos comestibles en los bosques de pino encino de Ixtlán de Juárez, Oaxaca. Revista Mexicana de Biodiversidad, 80, 521–534. https://doi.org/10.22201/ib.20078706e.2009.002.615
Gelardi, M. (2020). Diversity, biogeographic distribution, ecology, and ectomycorrhizal relationships of the edible porcini mushrooms (Boletus s. str., Boletaceae) worldwide: state of the art and an annotated checklist. In J. Pérez-Moreno, A. Guerin-Laguette, R. Flores Arzú, & F.Q. Yu (Eds.), Mushrooms, humans and nature in a changing World (pp. 223–271). Cham, Switzerland: Springer. https://doi.org/10.1007/978-3-030-37378-8_8
Kornerup, A., & Wanscher, J. H. (1978). Methuen handbook of colour. London: Methuen Publishing.
Largent, D., Johnson, D., & Watling, R. (1977). How identify mushrooms to genus III: microscopic features. Eureka, California: Mad River Press.
Leonardi, M., Marinho-Furtado, A. N., Comandini, O., Geml, J., & Rinaldi, A. C. (2020). Halimium as an ectomycorrhizal symbiont: new records and an appreciation of known fungal diversity. Mycological Progress, 19, 1495–1509. https://doi.org/10.1007/s11557-020-01641-0
Li, G. J., Hyde, K. D., Zhao, R. L., Hongsanan, S., Abdel-Aziz, F. A., Abdel-Wahab, M. A. et al. (2016). Fungal diversity notes 253-366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity, 78, 1–237. http://doi.org/10.1007/s13225-016-0366-9
Lodge, D. J., Ammirati, J. F., Dell, T. O., & Mueller, G. M. (2004). Terrestrial and lignicolous macrofungi: collecting and describing macrofungi. In G. Mueller, G. F. Bills, & M. S. Foster (Eds.), Biodiversity of Fungi. Inventary and monitoring methods (pp. 128–158). New York: Elsevier Academic Press.
Maddison, D. R., & Maddison, W. P. (2000). MacClade 4: analysis of phylogeny and character evolution. Sunderland, Massachussetts: Sinauer Associates.
Maddison, W. P., & Maddison, D. R. (2018). Mesquite: a modular system for evolutionary analysis. Version 3.40. http://www.mesquiteproject.org
Pierotti, A. (2015). Nomenclatural novelties: Cyanoboletus flavosanguineus (Lavorato & Simonini), in Pierotti, comb. nov. Index Fungorum, 263, 1.
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., & Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901-904. http://doi.org/10.1093/sysbio/syy032
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Sawar, S., Naaser, N., & Khalid, A. N. (2021). Cyanoboletus macroporus (Boletaceae), a new bolete species from Pakistani forests. Karstenia, 59, 78–87.
Snell, W. H., & Dick, E. A. (1958). Notes on Boletes X. A few miscellaneous discussions and a new species. Mycologia, 50, 57–65. https://doi.org/10.1080/00275514.1958.12024709
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22, 2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Thiers, B. M. (2024). Index Herbariorum. Retrieved on March 03rd, 2024 from: https://sweetgum.nybg.org/science/ih/
Vizzini, A. (2014). Nomenclatural novelties: Cyanoboletus Gelardi, Vizzini & Simonini, gen. nov. Index Fungorum, 176, 1.
Wu, G., Feng, B., Xu, J. P., Zhu, X. T., Li, Y. C., Zeng, N. K., Hosen, M. I., & Yang, Z. L. (2014). Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Diversity, 69, 93–115. https://doi.org/10.1007/s13225-014-0283-8
Wu, G., Li, Y. C., Zhu, X. T., Zhao, K., Han, L. H, Cui, Y. Y., Li, F., Xu, J. P., & Yang, Z. L. (2016). One hundred noteworthy boletes from China. Fungal Diversity, 81, 25–188. https://doi.org/10.1007/s13225-016-0375-8