Sergio I. Salazar-Vallejo a, * Víctor M. Conde-Vela b, Daniel A. López-Sánchez a
a El Colegio de la Frontera Sur, Departamento de Sistemática y Ecología Acuática, Ave. Centenario Km 5.5, Chetumal, Quintana Roo, México
b Department of Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th St. & Constitution Ave. NW, Washington, DC, 20560, USA
*Corresponding author: ssalazar@ecosur.mx (S.I. Salazar-Vallejo)
Received: 17 January 2024; accepted: 29 April 2024
Abstract
Aaron Treadwell described Lagisca crassa from Punta Arenas, Chile 100 years ago, based on an incomplete specimen. The species was later regarded as belonging in Eunoe Malmgren, 1865, but the species has not been found after its original description. The rediscovery of specimens collected in Punta Arenas has allowed us to evaluate its morphology to clarify some incompletely known features. Further, a comparison of the holotype of L. crassa with specimens of Hermadion magalhaensi Kinberg, 1856, led us to conclude these 2 species-group names are synonyms. We provide additional information and illustrations, and make some remarks on Eunoe and Hermadion Kinberg, 1856.
Keywords: Cephalic peaks; Anterior eyes; Lagisca; Hermadion; Antarctic Ocean
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Redescubrimiento de Eunoe crassa y aclaración de algunos atributos morfológicos (Annelida: Aphroditiformia: Polynoidae)
Resumen
Aarón Treadwell describió Lagisca crassa de Punta Arenas, Chile hace 100 años con un ejemplar incompleto. Luego la especie fue considerada como parte de Eunoe Malmgren, 1865, pero no fue recolectada de nuevo después de su descripción original. El redescubrimiento de ejemplares recolectados en Punta Arenas nos permitió evaluar su morfología y aclarar algunos atributos poco conocidos. Además, la comparación del material tipo de L. crassa con ejemplares de Hermadion magalhaensi Kinberg, 1856, nos hizo concluir que estas 2 especies nominales son sinónimas. En esta contribución proporcionamos información e ilustraciones adicionales y hacemos algunos comentarios sobre los géneros Eunoe y Hermadion Kinberg, 1856.
Palabras clave: Picos cefálicos; Ojos anteriores; Lagisca; Hermadion; Oceáno Antártico
Introduction
The family Polynoidae Kinberg, 1856 is one of the largest groups of marine annelids, and although there is some discrepancy about the generic definition for several taxa, and many synonyms were introduced without revisions, or after using a rather wide definition for genera, Polynoidae includes 12-13% of all polychaete genera, and about 8% or all polychaete species. Thus, Polynoidae would include almost 180 genera and about 900 species in some sources (Pamungkas et al., 2019), or 167 genera and 870 species (Read & Fauchald, 1924), but the most relevant feature is that the number of new taxa proposed per year is still growing (Pamungkas et al., 2019). One problem in identifying marine scaleworms is that they often break in parts, detach their elytra, or both, and this is widespread in specimens from the intertidal to abyssal depths. For this reason, many species have been described based on incomplete specimens. Further, as indicated by Barnich and Fiege (2009), as a result of a low number of taxonomic publications or revisions, for many polynoid genera “neither the respective generic nor specific identification characters have been critically evaluated.” This is further complicated because about 30% of all polynoid species are only known after the original description (Hourdez, 2024 pers. comm.).
The polynoin genera Lagisca Malmgren, 1865 and Eunoe Malmgren, 1865 are very similar by having 15 pairs of elytra but final segments without elytra, notochaetae as thick as, or thicker than neurochaetae, never with pilose or capillary tips, neurochaetae without semilunar pockets, with tips uni- or bidentate, ventral cirri digitate, venter smooth (Fauchald, 1977). The main difference separating them is the type of neurochaetae; in Lagisca there are at least some bidentate ones, whereas in Eunoe all are unidentate. Pettibone (1963) regarded both, Eunoe and Lagisca as subgenera of Harmothoe Kinberg, 1856. Malmgren (1865: key) separated Lagisca from Harmothoe because of the extent of dorsal cover by elytra. In Lagisca, the last segments are exposed, whereas in Harmothoe they are always covered. These 2 genera are regarded as synonyms because besides both having bidentate neurochaetae, larger specimens of Harmothoe usually have the last segments exposed (Barnich et al., 2006). However, as indicated by Fauvel (1916), the diagnostic relevance of this feature implies there are 12-19 chaetigers uncovered, as in Hermadion Kinberg, 1856, against a few (up to 5) in Harmothoe and other genera.
On the other hand, Eunoe also resembles Hermadion Kinberg, 1856, another subgenus in Harmothoe after Pettibone (1963), which is regarded as distinct by Wehe (2006). They are similar to each other by having the same number of elytra, final segments without elytra, and notochaetae as thick as, or thicker than neurochaetae. The main differences between them are that Eunoe species have less segments (40 vs. 50 or more), and neurochaetae are all unidentate in Eunoe, whereas they were regarded as uni- or bidentate in Hermadion. However, Bock et al. (2010) revised Hermadion, redefined the genus, and concluded it is monotypic, with H. magalhaensi Kinberg, 1856, as its type species. They did not provide an emended diagnosis but after their key, the diagnostic features would include body short, with up to 50 segments, prostomium without cephalic peaks, anterior eyes towards anterior margin (after figure), notochaetae with blunt tips, and neurochaetae denticulate, without semilunar pockets.
Hartman (1938) studied the holotype of Lagisca crassa and although she hesitated about its placement in Eunoe Malmgren, 1865, she completed the original description, corrected some details and included illustrations for the prostomium, 1 parapodium, and tips of 1 notochaeta and 2 neurochaetae. The prostomium has the anterior eyes ventral, under anterior prostomial margin, and chaetae were depicted with better definition of their fine details; she also indicated that palps, antennae and dorsal cirri were smooth, and that aciculae are exposed. Later, Hartman (1956, 1959) listed Treadwell’s species in Eunoe and hence confirmed the new combination.
Rozbaczylo (1985) noted that 3 species of Eunoe had been recorded for Chile: E. crassa (Treadwell, 1924), E. opalina M’Intosh, 1885, and E. rhizoicola Hartmann-Schröder, 1962. However, regarding E. crassa, after the original description, the species has been listed for Chile by Wesenberg-Lund (1962) but no additional specimens have been found.
On the other hand, Hermadion magalhaensi has more records for Chile, but only the original description (and the one for its junior synonym H. longicirratus Kinberg, 1856, plus 2 figures by Fauvel [1916]) has been illustrated, with all other records only listing the species in several Chilean localities from the intertidal to 200 m water depth (Rozbaczylo, 1985).
In this contribution, we document the discovery of some specimens of Eunoe crassa (Treadwell, 1924), collected in the type locality, and deposited in the University of Miami Voss Museum of Marine Invertebrates collection. Because the specimens are well-preserved, some remarks are introduced in the diagnosis of the involved genera, and the diagnostic features are clarified, explained, and accompanied by some illustrations. We also conclude, after the study of the holotype of E. crassa, that it is a junior synonym of H. magalhaensi.
Materials and methods
During part of the cruise 23 of the USNS Eltanin, some specimens were collected in Punta Arenas, Chile. They were deposited in the University of Miami Voss Museum of Marine Invertebrates (UMML). Additional specimens for comparison of H. magalhaensi (USNM 57798) and the holotype of L. crassa Treadwell, 1924 (USNM 19101) were examined at the National Museum of Natural History, Smithsonian Institution.
Specimens were observed with stereomicroscopes. Some detached elytra, parapodia and chaetae were observed in compound microscopes. Digital photos were stacked with HeliconFocus8, and plates were prepared with PaintShopPro and Photoshop CS.
Results
Order Phyllodocida Dales, 1962
Suborder Aphroditiformia Levinsen, 1883
Family Polynoidae Kinberg, 1856
Subfamily Polynoinae Kinberg, 1856
Eunoe Malmgren, 1865
Eunoe Malmgren, 1865: 61 (key, diagn.); Fauvel, 1923: 50; Uschakov, 1955: 147; Uschakov, 1965: 127 (diagn., key); Fauchald, 1977: 65; Barnich & Fiege, 2003: 29; Wehe. 2006: 125; Barnich & Fiege, 2010: 4.
Harmothoe (Eunoe): Pettibone, 1963: 34.
Type species. Polynoe nodosa Sars, 1861, by subsequent designation (Uschakov, 1955: 147; Uschakov, 1965: 128; Pettibone, 1963: 34; Jirkov, 2001: 145).
Diagnosis (slightly modified after Barnich and Fiege [2010]). Body depressed, short, with up to 50 segments; dorsum more or less covered by elytra or short posterior region uncovered. Fifteen pairs of elytra on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, and 32. Prostomium with or without distinct cephalic peaks and 3 antennae; lateral antennae inserted ventrally to median antenna. Anterior pair of eyes dorsolateral at widest part of prostomium, posterior pair dorsal near hind margin. Parapodia with elongate acicular lobes with both aciculae with tips exposed; neuropodia with a supra-acicular process. Notochaetae stout with distinct rows of spines, tips blunt. Neurochaetae more numerous and thinner, with distinct rows of spines distally and exclusively unidentate tips.
Remarks
Eunoe Malmgren, 1865 includes 46 species-group names distributed along all oceans from the intertidal to abyssal depths (Read & Fauchald, 2024). Malmgren (1865) included a key to genera and the diagnostic features for Eunoe were 15 pairs of elytra, covering dorsum, less than 45 segments; lateral antennae subventral; notochaetae with transverse rows of spines; neurochaetae unidentate with tips falcate, thinner than notochaetae. The same features were completed for the diagnosis (Malmgren, 1865).
There are no keys for identifying all Eunoe species. Species have been sorted out after the position of the anterior eyes (under anterior margin vs. median prostomial area), palp surface (papillose vs. spinulose), elytral features (pigmentation, fimbriae, macrotubercles), tips of notochaetae (tapered, mucronate, elongate), tips of neurochaetae (acute, swollen), and size or extent of subdistal denticulate region (short or long). Jimi et al. (2021) described a dimorphic species, and noted that after some molecular indicators, 2 groups of species can be recognized in Eunoe.
Malmgren (1865) included 2 species in Eunoe: E. oerstedi (Fig. 3A-D, in his plate 8), a replacement name for Lepidonote (sic) scabra Örsted, 1843, and the new combination of E. nodosa (Sars, 1861) for Polynoe nodosa Sars, 1861 (Fig. 4A-D in his plate 8). The main difference between these 2 species is the type of macrotubercles because in E. nodosa they have granulose tips, whereas they are spiny in E. oerstedi. On the other hand, the replacement name, E. oerstedi was unnecessary because there was no homonymy (ICZN, 1999, Art. 52.2) or matching combinations, but it has become accepted in recent publications and redescriptions (Barnich & Fiege, 2010; Pettibone, 1954, 1963).
The generic diagnosis included above indicates that prostomium has or lacks cephalic peaks. The type species, E. nodosa (Sars, 1861) has cephalic peaks “rather inconspicuous” (Barnich & Fiege, 2010).
On the other hand, the above diagnosis, slightly modified after Barnich and Fiege (2010), leaves out the species with eyes present towards the anterior prostomial region, as in E. crassa (Treadwell, 1924), not along the widest prostomial area. Other species having anterior eyes displaced anteriorly include E. alvinella Pettibone, 1989; E. barbata Moore, 1910; E. clarki Pettibone, 1951; E. hubrechti (McIntosh, 1900); E. papillosa Amaral & Nonato, 1982; E. rhizoicola Hartmann-Schröder, 1962; E. senta (Moore, 1902); E. spinosa Imajima, 1997 and E. subtruncata Annenkova, 1937. Further, the only species having anterior eyes displaced anteriorly and directed ventrally are E. barbata, E. clarki, E. rhizoicola, E. senta, and E. spinicirris. Another alternative, which should be based upon the study of type materials, would be to transfer these species to Hermadion, but this is beyond our current objectives.
The study of the type material of Lagisca crassa Treadwell, 1924 allowed us to conclude it belongs in Hermadion, and that it is a junior synonym of H. magalhaensi Kinberg, 1856, as redescribed elsewhere (Bock et al., 2010).
Hermadion Kinberg, 1856
Hermadion Kinberg, 1856: 386; Kinberg, 1858: 22; Baird, 1865: 196; Fauchald, 1977: 62, Bock et al., 2010: 46.
Type species. Hermadion magalhaensi Kinberg, 1856 by subsequent designation (Hartman, 1959: 79).
Diagnosis. Body depressed, short, with up to 50 segments, posterior region without elytra. Fifteen pairs of elytra on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, and 32. Prostomium without cephalic peaks, and 3 antennae, lateral antennae inserted ventrally to median one. Anterior pair of eyes ventrolateral, posterior pair towards hind margin. Parapodia with elongate acicular lobes with acicular tips exposed; neuropodia without supracicular process. Notochaetae stout with distinct rows of spines, tips blunt. Neurochaetae thinner, with distinct rows of spines distally and only unidentate tips.
Remarks
Hermadion Kinberg, 1856 is currently regarded as a monotypic genus (Bock et al., 2010). If the species listed above become regarded as members of Hermadion, then the potential diagnostic features would be centered in papillation of dorsal cirri and elytral ornamentation (fimbriae, and macro- and microtubercles); however, as indicated above, revising these species is beyond our current objectives.
Hermadion magalhaensi Kinberg, 1856
Figs. 1-4
Hermadion magalhaensi Kinberg, 1856: 386; Kinberg, 1858: 22, Pl. 6, Fig. 32; Ehlers, 1897: 15-16; Gravier, 1911: 86-87; Fauvel, 1916: 423-426, Pl. 8, Figs 10, 11; Augener, 1932: 13-14; Hartman, 1964: 35-37 (diagn., syn.); Averintsev, 1972: 123; Bock et al., 2010: 50-53 (redescr., syn.).
Lagisca crassa Treadwell, 1924: 1-3, 4 figs; Wesenberg-Lund, 1962: 26 (list).
Eunoe (?) crassa: Hartman, 1938: 119-120, Fig. 38b-e.
Eunoe crassa: Hartman, 1956: 261, 265 (list); Hartman, 1959: 67 (list).
Diagnosis. Hermadion with elytra without fimbriae, surface covered by short microtubercles, round and elongate spine-like; dorsal cirri with small papillae, tips mucronate, smooth.
Taxonomic summary
Type material. Punta Arenas, Chile. Holotype of Lagisca crassa Treadwell, 1924 (USNM 19101), Punta Arenas, Chile, 1923, F.F. Felippone, coll. (depth unknown).
Additional material. Punta Arenas, Chile. Four specimens (UMML), USNS Eltanin, Cruise 23, Sta. P4-3 (53°11’ S, 70°50’ W), shore collection, by hand, 0.8-1.6 km south of commercial pier, 30 Mar. 1966, fixed in isopropyl alcohol, McSween, coll. 11 specimens (USNM 57798), Cobble Beach, Magellanes, 6 May 1965, J. Mohr, coll.
Holotype of Lagisca crassa. The holotype (USNM 19101) is posteriorly incomplete, 7.3 mm long, 2.4 mm wide, 32 segments (Fig. 1A, C). Most elytra, cephalic appendages and dorsal cirri detached, some parapodia previously dissected, pharynx everted.

Prostomium longer than wide (Fig. 4F); eyes almost faded out, anterior eyes anteroventral (Fig. 1F). Ceratophore of median antenna with a V-shaped depression; ceratostyle missing. Lateral antenna ventral, ceratophores about half as wide as median one; ceratostyles 1.5x longer than ceratophores (Fig. 1F). Palps lost.
Tentacular cirri with chaetae; cirrostyles distally incomplete, slightly longer than cirrophores (Fig. 1F). Facial tubercle not visible dorsally.
Pharynx fully exposed (Fig. 1F, G); no pigments observed, slightly expanded distally; 9 pairs of marginal papillae. Jaws dark brown (Fig. 1G), blunt tips, without accessory denticles.
Elytra pale, non-fimbriate (Fig. 1B, C), with eccentric insertions. Surface almost fully covered with microtubercles; microtubercles rounded, rod-like, or distally truncate.
Parapodia biramous from segment 2. Few dorsal cirri remain attached, all without tips (Fig. 1D). Both notacicular and neuracicular lobes projected, tips of aciculae exposed (Fig. 1D, E). Ventral cirri tapered, reaching base of neuracicular lobe (Fig. 1D, E). Nephridial lobes blunt, present from segment 9 throughout body.
Notochaetae light brown, of different sizes (Fig. 1H, I), each blunt, with series of denticles, margin finely spinulose, tips delicately bent, entire (Fig. 1J, K). Neurochaetae light brown to transparent, subdistally expanded (Fig. 1L-O), with rows of denticles leaving tip smooth (Fig. 1P, Q); tip falcate, unidentate (Fig. 1P, Q).
Posterior end lost (Fig. 1A).
Additional material. The UMML and some USNM specimens were complete, body wall brittle, most elytra detached, some cephalic appendages and dorsal cirri lost, some with some portions removed likely after predatory attacks, especially along anterior region including right parapodia (Fig. 2), or right lateral antenna (Fig. 3B). Some specimens bent laterally, others bent ventrally, 2 with pharynx exposed. Body 35-47 mm long, 9-17 mm wide, 43-47 segments. Elytra overlapping laterally leaving middorsal area exposed in some specimens (Fig. 2A), fully covering it in others (Fig. 4A, B).
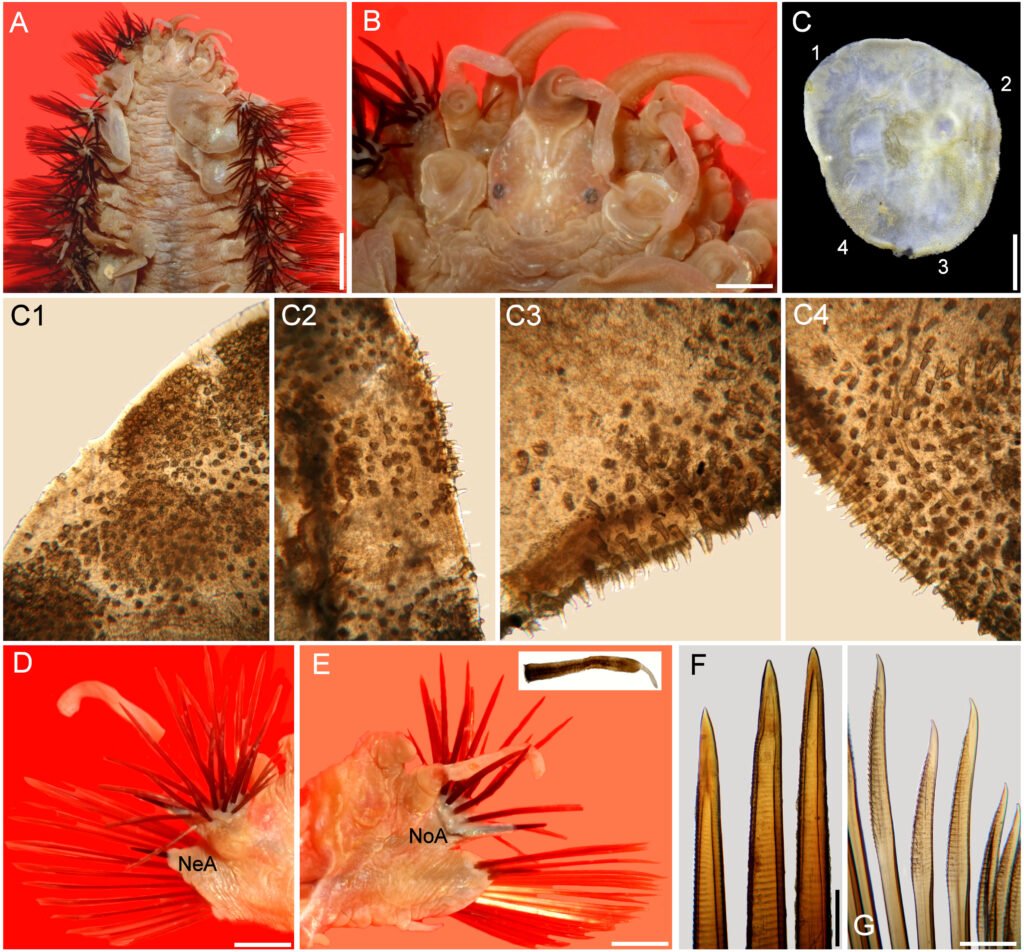
Figure 2. Hermadion magalhaensi Kinberg, 1856, topotype specimen (UMML). A, Anterior región, dorsal view; B, anterior end, dorsal view; C, right elytron 6, seen from above (1-4: sections enlarged in C1-C4); D, chaetiger 18, right parapodium, anterior view (NeA, neuracicular lobe); E, same, posterior view (inset: dorsal cirrus; NoA, notacicular lobe); F, tips of notochaetae; G, tips of neurochaetae. Scale bars: A, 2.1 mm; B, 0.6 mm; C, 1.1 mm; D, E, 1 mm; F, G, 180 µm.
Prostomium longer than wide. Eyes black, anterior eyes ventrolateral, not visible dorsally (Fig. 2B), better perceived in frontal view (Fig. 3A, B). Antennae and cirri cylindrical, tips mucronate, blunt. Median antenna with ceratophore forming a V-shaped depression, about 4 times wider than ceratostyle, ceratostyle about as long as prostomium. Lateral antennae ventral, ceratophores about half as wide as median one; ceratostyles lost. Palps thick, short, about as long as median antennae, finely papillate, but papillae not arranged in rows.
Tentacular cirri with cirrophores fused, with chaetae exposed; cirrostyles cylindrical mucronate. Facial tubercle pale, not visible dorsally, better defined after pharynx is exposed.
Elytra barely pigmented, non-fimbriate (Figs 2C, 4D), with variable amount of sediment particles. Surface covered by abundant microtubercles (Fig. 4D), small globular along anterior regions (Fig. 3C1, 2), progressively longer along posterior region, projected beyond elytral margin (Figs. 3C3, 4; 4E). Other specimens with a diffuse spot surrounding central area. Insertion area eccentric, displaced anteriorly and laterally, to the right in right elytra, to the left in left ones.

Figure 3. Hermadion magalhaensi Kinberg, 1856, topotype specimen (UMML). A, Anterior región and pharynx, dorsal view; B, anterior end, frontal view; C, pharynx opening, frontal view (Pa, papilla). Scale bars: A, 1 mm; B, 0.6 mm; C, 0.9 mm.
Parapodia biramous from segment 2. Dorsal cirri finely papillate, papillae not arranged in rows, tip smooth. Notopodia with dorsal cirri cylindrical, tip mucronate (Fig. 2D, E). Notacicular lobe projected, aciculae exposed (Fig. 4F, G). Neuropodia with neuropodial lobe projected, rarely with a long prechaetal subacicular lobe (Fig. 4G). Neuracicular lobe projected, aciculae exposed (Fig. 4F, G). Ventral cirri tapered, short, reaching base of neuracicular lobe. Nephridial lobes blunt, short, present from chaetiger 8-9, continued along body.
Notochaetae dark brown, abundant, roughly verticillate, each blunt, with series of denticles, margin finely spinulose, tips delicately bent, entire (Figs. 2F; 4H, I). Neurochaetae brownish, subdistally expanded, with rows of denticles leaving tip smooth; tip falcate, unidentate (Figs. 2G; 4J-M).
The pharynx is fully exposed; it is 9 mm long in a 46 mm long specimen (Fig. 3A). The outer surface looks maculate but the spots correspond with adsorbed crystals on the surface. The pharynx tube is slightly expanded distally, its margins are eroded and only the lateral papillae are left after erosion of most marginal integument (Fig. 3C); it was described with 9 pairs of marginal papillae. Jaws dark brown, tips blunt, without accessory denticles (Fig. 3C).
Posterior end tapered (Fig. 4C); pygidium with anus terminal; anal cirri short, resembling dorsal cirri.
Remarks
Hermadion magalhaensi Kinberg, 1856 and H. longicirratus Kinberg, 1856 were both described from the same locality and depth in Saint York Bay, Magellan Strait. The main differences between these species were that H. magalhaensi has smooth elytra, and smooth notochaetae, whereas H. longicirratus has elytra minutely tuberculate and spiny notochaetae; the former species was based on a 52 mm long specimen, whereas the latter on a 14 mm long specimen. The size difference might explain some features present in the smaller specimen and lost after abrasion in the larger specimen. However, the microtubercles in elytra might accumulate sediment and look smooth if they are not carefully cleaned. Fauvel (1916: 425) studied several specimens of different size and concluded H. magalhaensi and H. longicirratus were the same species.

Figure 4. Hermadion magalhaensi Kinberg, 1856, topotype specimen (USNM 57798). A, Whole specimen, dorsal view; B, anterior end, dorsal view; C, posterior end, dorsal view; D, right elytron from middle segment, seen from above; E, microtubercles from elytral margin; F, right cirrigerous chaetiger from middle segment, anterior view; G, right elytrigerous chaetiger from middle segment, anterior view; H, I, tips of notochaetae from middle segment; J, K, tips of supra-acicular neurochaetae from middle segment; L, M, tips of sub-acicular neurochaetae from middle segment. Scale bars: A, 3 mm; B, C, 1 mm; D, F, G, 0.5 mm; E, 0.1 mm; H-M, 50 μm.
Treadwell (1924) described Lagisca crassa from Punta Arenas, Chile based on an incomplete specimen, without most of its elytra, and included figures for the anterior end, 1 cirrigerous parapodium, and tips of 1 notochaetae (tapered), and 1 neurochaetae (unidentate), but no elytra were illustrated. Ceratostyles of antennae and dorsal cirrostyles were shown with a subdistal brown ring but were not subdistally expanded. The median antenna is longer than laterals, its base marks a deep V-shaped depression over prostomium, and the parapodium shows acicular lobes projected, but tips of aciculae were not emergent. The pharynx was indicated as having 9 pairs of marginal papillae, but no details of the jaws were provided.
On the other hand, what has been regarded as E. crassa and E. rhizoicola Hartmann-Schröder, 1962 are the 2 only species described and recorded from shallow water depths in Chile. The latter species was also described from Punta Arenas, with a 21 mm long specimen. These 2 species are very similar by having anterior eyes displaced anteriorly, similar types of noto- and neurochaetae, and dorsal cirri with black bands. They differ because in E. crassa the dorsal cirri have a single subdistal black band, against 2 in E. rhizoicola, and its tip is short, whereas it is longer in E. rhizoicola. The main difference is in the presence of fimbriae; there are no fimbriae in E. crassa, whereas E. rhizoicola has some short filaments along posterior margins. It is likely that E. rhizoicola is another junior synonym of H. magalhaensi because it resembles H. longicirratus in having longer dorsal cirri, but this might be a size dependent feature, becoming relatively shorter in larger specimens. Further, H. magalhaensi has been found living in Macrocystis rhizoids (Pratt, 1901), which was the habitat also for E. rhizoicola.
We think that the main reason for the confusion regarding the affinities between what was described as L. crassa and H. magalhaensi is because there were only 1 set of illustrations of the species (Kinberg, 1858), and since during many years, the proposals for new records or new species did not include the study of type or topotype specimens (Fauchald, 1989).
Distribution. Originally described from Puntarenas, Chile, in shallow depths (0-200 m), it ranges along subantarctic localities including the Falkland and Kerguelen Islands.
Acknowledgments
Geoff Read, Igor Jirkov and Oscar Díaz-Díaz kindly provided useful publications. William Moser found some additional field data for the Eltanin station where the specimens were found. Stéhane Hourdez and an anonymous referee carefully read this contribution and suggested several important modifications. The technical editorial issues were masterfully made by María Antonieta Arizmendi.
References
Amaral, A. C., & Nonato, E. F. (1982). Anelideos poliquetos da costa brasileira, 3. Aphroditidae e Polynoidae. Brazilia, Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Annenkova, N. P. (1937). Fauna Polychaeta severnoii chasti Yaponskogo Morya. Issledovaniya Morei SSSR, 23, 139–216.
Augener, H. (1932). Antarktische und Antiboreale Polychaeten nebst einer Hirudinee. Scientific Results of the Norwegian Antarctic Expeditions 1927-1928 et sqq., instituted and financed by Consul Lars Christensen, 9, 1–85.
Averintsev, V. G. (1972). Donnye mnogotchetinkovye chervi Errantia Antarktiki i Subantarktiki no materialam sovetskoi Antarktitcheskoi Exspeditsii. Issledovaniya Fauny Morei, 11(19). Resul’taty biologicheskikh issledovanii Sovetskikh Antarktitseskikh Exspeditsii, 5, 85–293.
Baird, W. (1865). Contributions towards a monograph of the species of Annelides belonging to the Aphroditacea, containg a list of the known species, and a description of some new species contained in the National Collection of the British Museum. Journal of the Linnean Society, Zoology, 8, 172–202. https://doi.org/10.1111/j.1096-3642.1865.tb02438.x
Barnich, R., & Fiege, D. (2003). The Aphroditoidea (Annelida: Polychaeta) of the Mediterranean Sea. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft Frankfurt am Main, 559, 1–167.
Barnich, R., & Fiege, D. (2009). Revision of the genus Harmothoe Kinberg, 1856 (Polychaeta: Polynoidae) in the Northeast Atlantic. Zootaxa, 2104, 1–76. https://doi.org/10.11646/zoo
taxa.2104.1.1
Barnich, R., & Fiege, D. (2010). On the distinction of Harmothoe globifera (G.O. Sars, 1873) and some other easily confused polynoids in the NE Atlantic, with the description of a new species of Acanthicolepis Norman in McIntosh, 1900 (Polychaeta, Polynoidae). Zootaxa, 2525, 1–18. https://doi.org/10.11646/zootaxa.2525.1.1
Barnich, R., Fiege, D., Micaletto, G., & Gambi, M. C. (2006). Redescription of Harmothoe spinosa Kinberg, 1856 (Polychaeta: Polynoidae) and related species from Subantarctic and Antarctic Waters, with the erection of a new genus. Journal of Natural History, 40, 33–75. https://doi.org/10.1080/00222930500445044
Bock, G., Fiege, D., & Barnich, R. (2010). Revision of Hermadion Kinberg, 1856, with a redescription of Hermadion magalhaensi Kinberg, 1856, Adyte hialina (G.O. Sars, 1873) n. comb., and Neopolynoe acanellae (Verrill, 1881) n. comb. (Polychaeta: Polynoidae). Zootaxa, 2554, 45–61. https://doi.org/10.11646/zootaxa.2554.1.4
Dales, R. P. (1962). The polychaete stomodeum and the inter-relationships of the families of Polychaeta. Proceedings of the Zoological Society of London, 139, 389–428. https://doi.org/10.1111/j.1469-7998.1962.tb01837.x
Ehlers, E. (1897). Hamburger Magalhaensischen Sammelreise 1892/93 3(Bryozoen und Würmer). Polychaeten. Hamburg: Friederichsen & Co.
Fauchald, K. (1977). The polychaete worms: definitions and keys to the orders, families and genera. Natural History Museum of Los Angeles County, Science Series, 28, 1–188.
Fauchald, K. (1989). The second annual Riser lecture: eclecticism and the study of polychaetes. Proceedings of the Biological Society of Washington, 102, 742–752.
Fauvel, P. (1916). Annélides polychètes des Iles Falkland recueillies par M. Rupert Vallentin Esq (1902-1910). Archives de Zoologie Expérimentale et Générale, 55, 417–482. https://doi.org/10.5962/bhl.part.11511
Fauvel, P. (1923). Polychètes errantes. Faune de France, 5,1–488.
Gravier, C. (1911). Deuxième Expédition Antarctique Française (1908-1910), commandé par le Dr. Jean Charcot. Annélides polychètes. Paris: Masson et Cie.
Hartman, O. (1938). The types of the polychaete worms of the families Polynoidae and Polyodontidae in the United States National Museum and the description of a new genus. Proceedings of the United States National Museum, 86, 3046, 107–134. https://doi.org/10.5479/si.00963801.86-3046.107
Hartman, O. (1956). Polychaetous annelids erected by Treadwell, 1891 to 1948, together with a brief chronology. Bulletin of the American Museum of Natural History, 109, 239–310.
Hartman, O. (1959). Catalogue of the polychaetous annelids of the World. Allan Hancock Foundation Publications, Occasional Paper, 23, 1–628.
Hartman, O. (1964). Polychaeta Errantia of Antarctica. Antarctic Research Series, 3, 1–131. https://doi.org/10.1029/ar003
Hartmann-Schröder, G. (1962). Zur Kenntnis des Eulitorals der chilenischen Pazifikküste und der argentinischen Küste Südpatagoniens unter besonderer Berücksichtigung der Polychaeten und Ostracoden. Die Polychaeten des Eulitorals. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut, 60, 57–270.
Imajima, M. (1997). Polychaetous annelids from Sagami Bay and Sagami Sea collected by the Emperor Showa of Japan and deposited at the Showa Memorial Institute, National Science Museum, Tokyo. Families Polynoidae and Acoetidae. National Science Museum Monographs, 13,1–131.
ICZN (International Commission of Zoological Nomenclature). (1999). International Code of Zoological Nomenclature. London, Natural History Museum. Available at: www.code.iczn.org
Jimi, N., Hookabe, N., Moritaki, T., Kimura, T., & Imura, S. (2021). First evidence of male dwarfism in scale worms: A new species of Polynoidae (Annelida) from hermit crab and molluscan shells. Journal of Zoological Systematics and Evolutionary Research, 59,801–818. https://doi.org/10.1111/jzs.12463
Jirkov, I. A. (2001). Polikhety Severnogo Ledovitogo Okeana. Tromsø, Akvaplan-Niva.
Kinberg, J. G. H. (1856). Nya slägten och arter af Annelider. Öfversigt af Kongliga Vetenskaps-Akademiens Förhhandlingar Stockholm, 12, 381–388.
Kinberg, J. G. H. (1858). Kongliga Svenska Fregatten Eugenies resa Omkring Jorden under befäl af C.A. Virgin, Ånen 1851-1853, Vetenskapliga Iakttagelser, Zoologi, 3. Annulater. Stockholm.
Levinsen, G. M. R. (1883). Systematisk-geografisk Oversigt over de nordiske Annulata, Gephyrea, Chaetognathi og Balanoglossi. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i Kjøbenhavn, 1882, 160–251. https://doi.org/10.5962/bhl.title.16117
Malmgren, A. J. (1865). Nordiska Hafs-Annulater. Öfversigt af Kongl. Vetenskaps-Akademiens Förhandlingar, 22,51–110,
M’Intosh, W. C. (1885). Report on the Annelida Polychaeta collected by H.M.S. Challenger during the years 1873-1876. Reports on the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873–76, Zoology, 12,i-xxxvi.
McIntosh, W. C. (1900). A monograph of British Annelids, 1(2) Polychaeta Amphinomidae to Sigalionidae. London: Ray Society of London.
Moore, J. P. (1902). Descriptions of some new Polynoidae, with a list of other Polychaeta from North Greenland waters. Proceedings of the Academy of Natural Sciences of Philadelphia, 54, 258–278.
Moore, J. P. (1910). The polychaetous annelids dredged by the U.S.S. “Albatross” off the coast of Southern California in 1904: 2. Polynoidae, Aphroditidae and Segalionidae (sic). Proceedings of the Academy of Natural Sciences of Philadelphia, 62, 328–402.
Örsted, A. S. (1843). Grönlands Annulata dorsibranchiata. Det Kongelige Danske videnskabernes selskabs. Naturviden-
skabelige og Mathematiske Afhandlinger, 10, 153–216.
Pamungkas, J., Glasby, C. J., Read, G. B., Wilson, S. P., & Costello, M. J. (2019). Progress and perspectives in the discovery of polychaete worms (Annelida) of the world. Helgolander Marine Research, 73, 4. https://doi.org/10.1186/s10152-019-0524-z
Pettibone, M. H. (1951). A new species of polychaete worm of the family Polynoidae from Point Barrow, Alaska. Journal of the Washington Academy of Sciences, 41,44–45.
Pettibone, M. H. (1954). Marine polychaete worms from Point Barrow, Alaska, with additional records from the North Atlantic and North Pacific. Proceedings of the United States National Museum, 103, 203–356. https://doi.org/10.5479/si.00963801.103-3324.203
Pettibone, M. H. (1963). Marine polychaete worms of the New England región, 1. Families Aphroditidae through Trochochaetidae. Bulletin, United States National Museum, 227, 1–356. https://doi.org/10.5479/si.03629236.227.1
Pettibone, M. H. (1989). Two new species of Harmothoinae (Polychaeta: Polynoidae) from the East Pacific Rise, collected by Alvin dives 2000 and 2003. Proceedings of the Biological Society of Washington, 102,305–310.
Pratt, E. M. (1901). A collection of Polychaeta from the Falkland Islands. Manchester Memoirs, Memoirs and Proceeding of the Manchester Literary & Philosophical Society, 45, 1–18.
Read, G., & Fauchald, K. (Eds). (2024). World Polychaeta database. Eunoe Malmgren, 1865. Retrieved on January 17 2024, from: https://www.marinespecies.org/aphia.php?p=
taxdetails&id=129487
Rozbaczylo, N. (1985). Los anélidos poliquetos de Chile: Índice sinonímico y distribución geográfica de especies. Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Monografías Biológicas, 3, 1–284.
Sars, M. (1861). Om de ved Norges Kyster forekommende Arter af Annelideslaegten Polynoë. Forhandlinger i Videnskabs-Selskabet i Christiania, 1860, 54–62.
Treadwell, A. L. (1924). A new marine annelid from Chile. Proceedings of the United States National Museum, 65, 1–3. https://doi.org/10.5479/si.00963801.65-2536.1
Uschakov, P. V. (1955). Mnogotschetinkovye chervi Dal’ne-
vostochitchykh Morei, SSSR. Akademiya Nauk, Opredeliteli ro Faune SSSR, Isdavaemye Zoologicheskim Institutom, 56, 1–445.
Uschakov, P. V. (1965). Polychaeta of the Far Eastern Seas of the U.S.S.R. Zoological Institute of the Russian Academy of Sciences, Keys to the Fauna of the U.S.S.R., 56, 1–419.
Wehe, T. (2006). Revision of the scale worms (Polychaeta: Aphroditoidea) occurring in the seas surrounding the Arabian Peninsula, 1. Polynoidae. Fauna of Arabia, 22, 23–197.
Wesenberg-Lund, E. (1962). Reports of the Lund University Chile Expedition 1948-49, 43. Polychaeta Errantia. Lunds Universitets Årsskrift, neue folge, Series 2, 57, 1–139.




