Carlos Hiram Rábago-Quiroz a, Adrián Felipe González-Acosta b, *, Gorgonio Ruiz-Campos c, Jonathan Franco-López d, Juan Antonio García-Borbón a, Joaquín D. Magaña e
a Instituto Mexicano de Investigación en Pesca y Acuacultura Sustentables, Centro Regional de Investigación Acuícola y Pesquera, La Paz, Km. 1 Carretera Pichilingue s/n, Col. Esterito, 23020 La Paz, Baja California Sur, Mexico
b Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, Av. Instituto Politécnico Nacional s/n, Col. Playa Palo de Santa Rita, 23096 La Paz, Baja California Sur, Mexico
c Universidad Autónoma de Baja California, Facultad de Ciencias, Colección Ictiológica, Carretera Transpeninsular Ensenada-Tijuana Núm. 3917, Colonia Playitas, 22860 Ensenada, Baja California, Mexico
d Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, Laboratorio de Ecología, Av. De los Barrios Núm. 1, Colonia Los Reyes Ixtacala, 54090 Tlalnepantla, Estado de México, Mexico
e University of Belize, Belmopan Campus, P.O. Box 340, Cayo, Belize
*Corresponding author: aacosta@ipn.mx (A.F. González-Acosta)
Received: 20 June 2023; accepted: 21 February 2024
Abstract
We present an updated systematic checklist of fishes from the Bahía Magdalena-Almejas lagoon system (Mexico), including notes on taxonomy, zoogeography, and conservation status, supported by field samplings and the critical review of published and online data. The ichthyofauna is composed by 2 classes, 30 orders, 104 families, 240 genera and 416 species, mainly represented by the class Actinopterygii. Zoogeographically, the fishes showed highest affinity to the San Diego (87.2%), Cortez (81.4%) and Mexican (70%) provinces, reflecting the ancient relationship between the Californian and Tropical Eastern Pacific regions and the existence of a transitional zone in the Baja California Peninsula for fish assemblages from boreal-temperate and warm-tropical derivation, standing out the presence of the endemic Paraclinus magdalenae. The 91% of the ichthyofauna is included by the IUCN Red List as Lower Concern, Data Deficient and Not Evaluated, while Holacanthus clarionensis and Hippocampus ingens, are under Special Protection by the Mexican regulation.Ecologically, 233 species are marine-euryhaline and 177 marine-stenohaline, which preferentially inhabit soft (57%) and rocky (27.5%) bottoms; most species are demersal (60%), benthic (25%) and pelagic (14.4%). The updated checklist could improve the design and implementation of effective fishing regulation strategies and conservation programs for fishes inhabiting this coastal ecosystem.
Keywords: Fish diversity; Conservation status; Taxonomy; Mexican Pacific; Checklist
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Composición y zoogeografía de peces marino-estuarinos de un sistema lagunar en una zona de transición templado-tropical del Pacífico oriental
Resumen
Se presenta la lista sistemática actualizada de los peces del sistema lagunar Bahía-Magdalena-Almejas, con notas taxonómicas, zoogeografía y estado de conservación; basada en recolectas biológicas y la revisión crítica de datos publicados. La lista la integran 2 clases, 30 órdenes, 104 familias, 240 géneros y 416 especies, mayormente representados por Actinopterygii. La mayor afinidad zoogeográfica con las provincias de San Diego (87.2%), de Cortés (81.4%) y Mexicana (70%), resulta de la relación ancestral entre las regiones de California y Pacífico oriental tropical y debido a la existencia de una zona de transición en la península de Baja California, para conjuntos ícticos de derivación boreal-templada y subtropical-tropical, donde destaca el endémico Paraclinus magdalenae. De acuerdo con la Lista Roja de la UICN, 91% de la ictiofauna es de preocupación menor, datos deficientes y no evaluado; mientras que Holacanthus clarionensis e Hippocampus ingens, están bajo protección especial por la legislación mexicana.Ecológicamente, 233 especies son marino-eurihalinas y 177 marino-estenohalinas, habitan preferentemente sobre fondos suaves (57%) y rocosos (27.5%); 60% son demersales, 25% bentónicas y 14% y pelágicas. La lista sistemática actualizada permitirá formular una estrategia efectiva de regulación pesquera y programas de conservación en este ecosistema.
Palabras clave: Diversidad íctica; Estado de conservación; Taxonomía; Pacífico mexicano; Listado sistemático
Introduction
The western coast of the Baja California peninsula (BCP) is recognized as a biogeographic region with high fish diversity (Castro-Aguirre & Espinosa-Pérez, 2006; Hastings, 2000; Ruiz-Campos et al., 2010), and one of the most productive fishing areas worldwide (Finkbeiner & Basurto, 2015; Ojeda-Ruiz et al., 2018). Within this region, the Bahía Magdalena-Almejas (BMA) lagoon system represents the largest and the main fishing zone in the northwestern Mexican Pacific region, including the Baja California Sur state (Avendaño-Ibarra et al., 2004; Cota-Nieto et al., 2015; Ojeda-Ruiz et al., 2018).
The privileged geomorphology and geographic location of the BMA lagoon system, in the transition zone between the Californian and Tropical Eastern Pacific regions (Briggs, 1974; Castro-Aguirre & Espinosa-Pérez, 2006; Castro-Aguirre et al., 1992, 1993; González-Acosta, Ruiz-Campos et al., 2015; Hastings, 2000; Parrish et al.,1981; Ruiz-Campos et al., 2010), promote the existence of a large extension (17 million m2; Acosta-Velázquez & Ruiz-Luna, 2007) of mangrove biotopes that house a great diversity of fishes from temperate and tropical derivation (Etnoyer et al., 2004; Funes-Rodríguez et al., 2007; Hernández-Velasco et al., 2016; Malakoff, 2004; Whitmore et al., 2005). Thereby, plays an important role as the primary habitat or nursery grounds for feeding, spawn, and recruitment for young and adult of commercial fish species as well as for those subject to ecological conservation (Cota-Nieto et al., 2015; Hastings & Fischer, 2001); which mostly are representative of pelagic and demersal fish species that use these habitats alternating seasonality their abundances, generating critical links in the food chains between the lagoon system and the adjacent sea (González-Acosta, Ruiz-Campos et al., 2015).
The BMA lagoon system is the second most studied and richest fish area in BCP (Hinojosa-Medina et al., 2007), after Bahía de La Paz (González-Acosta, Balart et al., 2018; González-Acosta, Ruiz-Campos et al., 2015); on this basis, the fish fauna previously reported for BMA varies between 92 to 302 species (Castro-Aguirre et al., 1993; Galván-Magaña et al., 2000). However, given that the specific richness of this ecosystem has substantially increased since its fish fauna has been more deeply studied and new taxa are reported or formally described, this study aims to document and update the systematic checklist of the marine and estuarine fish species occurring in the BMA lagoon system, including notes on taxonomy, zoogeography, and conservation status.
Material and methods
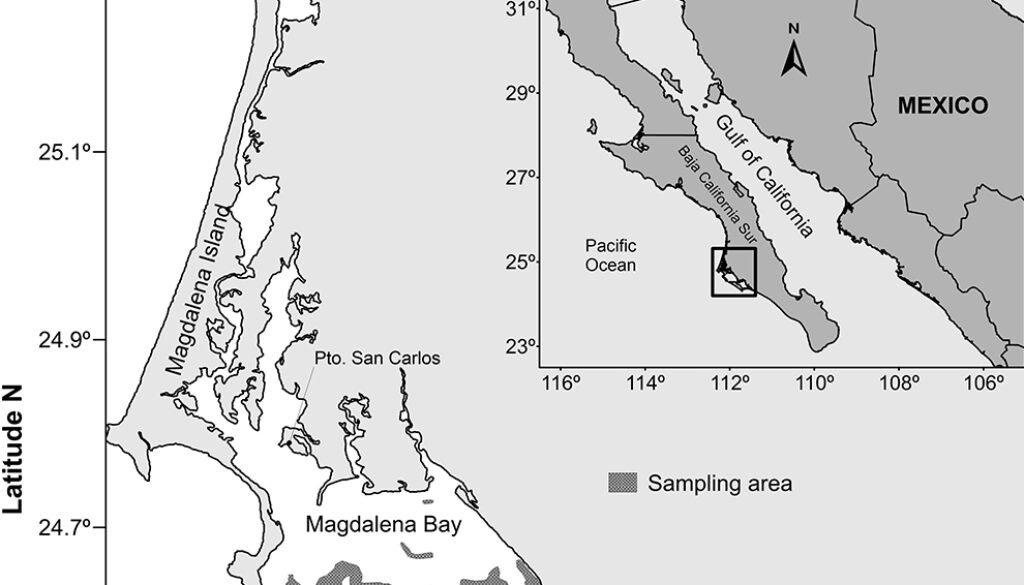
The BMA is an extensive estuarine lagoon system (114,600 ha) bordered by islands located parallel to the coast (Magdalena, Margarita and Creciente), coastal dunes, and extensive sandbars with sparse vegetation. This coastal ecosystem is located in the southwestern portion of the BCP (24°21’ – 24°46’ N, 110°30’- 112°15’ W; Fig. 1), which comprises 3 subsystems: the Northwestern zone (137 km2), characterized by the presence of negative estuaries (locally named “esteros”), marshes and channels with a mean depth of 3.6 m; the Central zone that comprises Bahía Magdalena (883 km2) with a mean depth of 12 – 15 m, which connects with the adjacent sea via a 5.6 km wide inlet (≥ 40 m deep); and the Southern portion comprised by Bahía Almejas (370 km2) that is characterized by shallow plains that are exposed at low tides, as well as a deep (~ 30 m) and wide (2-3 km) channel that communicates with BM, and empties into the sea through a shallow inlet (Álvarez-Borrego et al., 1975; Castro-Aguirre & Torres-Orozco, 1993; Lewis & Ebeling, 1971; Ojeda-Ruiz et al., 2018).
The hydrology of the BMA lagoon system is influenced during the Spring-Summer seasons by the cool California Current and from Autumn-Winter by the warm subtropical California Countercurrent with high evaporation rates in the shallow areas and high-water transport in deep zones of channels and lagoons, and upwellings in front of the mouth (Álvarez-Borrego et al., 1975; Avendaño-Ibarra et al., 2004; Funes-Rodríguez et al., 2007; Gómez-Valdez & Vélez-Muñoz, 1982; Lluch-Belda et al., 2000). The surface temperature ranges from 18 to 29 °C and the dissolved oxygen from 0.04 to 2.56 mg/l, tides are semi-diurnal. The salinity varies from 34 to 40 ups, due to the absence of rain and river runoffs; therefore, this lagoon system behaves throughout the year as a negative estuary (Álvarez-Borrego et al., 1975; Castro-Aguirre & Torres-Orozco, 1993), as is common in this arid region (Brusca et al., 2005; González-Acosta, Rabadán-Sotelo et al., 2015; González-Acosta, Ruiz-Campos et al., 2015).
The systematic checklist of fishes inhabiting the BMA lagoon system was based on fish specimens collected during the study period from March 2014 to June 2018 in several sites along this ecosystem, using a shrimp trawl net (16 -17 m long headline and 3.5 cm mesh size), deployed at 2 knots for 1 hour. All caught specimens were frozen and transported to the fish laboratory at Centro Regional de Investigación Acuícola y Pesquera-La Paz of the Instituto Mexicano de Investigación en Pesca y Acuacultura Sustentables (IMIPAS-CRIAP), to be fixed with 10% formalin and later preserved in 70% ethanol. The taxonomic identification was based on specialized taxonomic keys and fish catalogs (Allen & Robertson, 1994; Castro-Aguirre et al., 1999; Fischer et al., 1995; Love & Passarelli, 2020; Love et al., 2021), and online databases (e.g., Robertson & Allen, 2015). Some specimens of each species were housed as vouchers in the Fish Collection of the IMIPAS.
The systematic checklist also includes documented fish records (including larvae) compiled throughout the critical taxonomic review of previous and current checklist species published in specialized literature (Avendaño-Ibarra et al., 2004; Castro-Aguirre & Torres-Orozco, 1993; Castro-Aguirre et al., 1992; De la Cruz-Agüero et al., 1994; Funes-Rodríguez et al., 2007; Galván-Magaña et al., 2000; González-Acosta, Ruiz-Campos et al., 2015), and online databases of reference fish collections. The taxonomic arrangement (nomenclature and classification) of the systematic checklist follows to Page et al. (2013) and Fricke et al. (2023), respectively. The geographic distribution records for each species were confirmed in the online databases of Peces Costeros del Pacífico Oriental Tropical (Robertson & Allen, 2015), the Global Biodiversity Information Facility (GBIF, 2023), the Eschmeyer’s Catalog of Fishes (Fricke et al., 2023) and FISHBASE data (Froese & Pauly, 2023); thereby, the zoogeographic affinity of the fish fauna was determined following Briggs´s regionalization (Briggs, 1974), as well as by their distributional patterns based on Castro-Aguirre et al. (2005, 2006). Conservation status and ecology of the fish species here included were documented based on the Red List of Threatened Species of the International Union for Conservation of Nature (IUCN, 2022) and the Mexican Flora and Fauna Protection Regulation NOM-059-SEMARNAT-2010 (DOF, 2010, 2019), with complementary information obtained from published studies involving local fish fauna (Castro-Aguirre & Torres-Orozco, 1993; Castro-Aguirre et al., 1992, 1993; Fischer et al. 1995; Froese & Pauly, 2023; Funes-Rodríguez et al., 2007; González-Acosta, Ruiz-Campos et al., 2015; Love & Passarelli, 2020; Love et al., 2021; Robertson & Allen, 2015).
Results
The BMA fish fauna is composed of 2 classes, 30 orders, 104 families, 240 genera and 416 species (Table 1, Fig. 2). The class Chondrichthyes groups 45 species, 25 genera, 18 families, 8 orders, and the subclass Elasmobranchii, represented by 2 divisions: Selachii (sharks) composed by 5 orders, 7 families, 12 genera and 23 species, most of them corresponding to the order Carcharhiniformes (3 families, 8 genera and 18 species) and the family Carcharhinidae (Carcharhinus Blainville, 1816 and Sphyraena Artedi, 1793 with 4 species each); and Batomorphi (rays and skates) represented by 3 orders, 11 families,13 genera and 22 species, predominating the order Myliobatiformes (15 species, 8 genera and 7 families) and the family Urotrygonidae (2 genera and 7 species).
The class Actinopterygii encompassess 89.2% of the total fish fauna of the BMA lagoon system, represented by 22 orders, 86 families, 215 genera and 371 species, with the representativeness of the order Perciformes (45 families, 134 genera and 224 species); while the families: Sciaenidae (27 species), Haemulidae (22 species), Carangidae (18 species), Gobiidae (15 species), Serranidae (12 species), and Epinephelidae (11 species), comprise 46.9% of the order, 28.3% of the class and 25.2% of the total richness in this lagoon system. Fish larvae are represented by 76 species (20.5% of the total actinopterygian fishes) grouped in 58 genera, 36 families and 11 orders, most of the species correspond to the order Perciformes, of which Gobiidae and Labrisomidae are the most diverse families with 5 species each.
Based on the assessment of the zoogeographic affinity, the fish fauna of BMA lagoon system shows affinity to the Auletian (18 species, 4.3%), Oregonian (53 species, 13%), San Diego (362 species, 87.2%), Cortez (338 species, 81.4%), Mexican (290 species, 70%), Panamian (262 species, 63.1%), Galapagos (81 species, 20%), and Chilean-Peruvian (8 species, 1.9%) provinces. According to their reported distribution range, only the Magdalena blenny Paraclinus magdalenae Rosenblatt & Parr, 1969 (0.2%) is endemic, 38 species are circumtropical (9.2%), 4 amphipacific (1%), and 5 amphiamerican (1.2%).

Figure 1. Location of the Bahía Magdalena-Almejas lagoon system in the northeastern Pacific of Mexico. Sampling trawl fishing sites are indicated in grey.
Based on the Red List criteria of the IUCN (IUCN, 2022), 3 (0.7%) species are “Critically Endangered” (2 chondrichthyans: the Scalloped hammerhead, Sphyraena lewini (Grifit and Smith, 1834]) and the Great hammerhead, Sphyrna mokarran (Rüpell, 1832), and the Giant seabass Stereolepis gigas Ayres, 1859); 19 (4.6%) are “Vulnerable” (6 selachians, 6 batoids and 7 actinopterygians); 6 (1.5%) are “Endangered” (4 selachians and 2 actinopterygians); 10 (2.4%) are “Near Threatened” (3 selachians and 7 batoids); 17 (4.1%) are “Not Evaluated” (all actinopterygians); 21 (5.1%) are “Data Deficient”(1 selachian, 2 batoids and 18 actinopterygians); and 340 (81%) are of “Least Concern” (6 selachians, 6 batoids and 328 actinopterygians) (Table 1). According to the latest update of the NOM-059-SEMARNAT-2010 (DOF, 2019), 2 (0.5%) species are under “Special Protection (Pr)”: the Clarion angelfish Holacanthus clarionensis Gilbert, 1891 and the Pacific seashore Hippocampus ingens Girard, 1855.
| Table 1 Systematic list of estuarine-marine fishes of the Bahía Magdalena-Almejas lagoon system (México). | ||||||
| Taxa | ZA | IUCN | NOM | D | S | H |
| Class Chondrichthyes | ||||||
| Subclass Elasmobranchii | ||||||
| Order Heterodontiformes | ||||||
| Family Heterodontidae | ||||||
| Heterodontus francisci (Girard, 1855) | SD, C, M, P | DD | ES | S | B | |
| Heterodontus mexicanus Taylor & Castro-Aguirre, 1972 | SD, C, M, P | LC | ES | S | B | |
| Order Lamniformes | ||||||
| Family Lamnidae | ||||||
| Isurus oxyrinchus Rafinesque, 1810 | CT | EN | MA | MP | ||
| Order Carcharhiniformes | ||||||
| Family Triakidae | ||||||
| Mustelus albipinnis Castro-Aguirre, Antuna-Mendiola, González-Acosta & De la Cruz-Agüero, 2005 | SD, C | LC | ES | S | D | |
| Mustelus californicus Gill, 1864 | SD, C | LC | ES | S | D | |
| Mustelus henlei (Gill, 1863) | O, SD, C, M, P | LC | ES | S | D | |
| Mustelus lunulatus Jordan & Gilbert, 1882 | SD, C, M, P | LC | ES | S | D | |
| Triakis semifasciata Girard, 1855 | O, SD, C | LC | ES | S | D | |
| Family Carcharhinidae | ||||||
| Carcharhinus albimarginatus (Rüpell, 1837) | CT | VU | MA | BP | ||
| Carcharhinus leucas (Müller & Henle, 1839) | CT | VU | ES | S | D | |
| Carcharhinus limbatus (Müller & Henle, 1839) | CT | VU | ES | S | EP | |
| Carcharhinus obscurus (Lesueur, 1818) | CT | EN | MA | MP | ||
| Galeocerdo cuvier (Péron & Lesueur, 1822) | CT | NT | MA | EP | ||
| Nasolamia velox (Gilbert, 1898) | SD, C, M, P | EN | MA | D | ||
| Negaprion brevirostris (Poey, 1868) | AA | VU | ES | R | D | |
| Prionace glauca (Linnaeus, 1758) | SD, C, M, P | NT | MA | EP | ||
| Rhizoprionodon longurio (Jordan & Gilbert, 1882) | SD, C, M, P | VU | MA | S | D | |
| Family Sphyrnidae | ||||||
| Sphyrna lewini (Griffith & Smith, 1834) | CT | CR | MA | EP | ||
| Sphyrna mokarran (Rüpell, 1837) | CT | CR | MA | EP | ||
| Sphyrna tiburo (Linnaeus, 1758) | AA | EN | ES | R | D | |
| Sphyrna zygaena (Linnaeus, 1758) | CT | VU | MA | D | ||
| Order Squaliformes | ||||||
| Family Squalidae | ||||||
| Squalus suckleyi (Girard, 1854) | AP | LC | MA | EP | ||
| Order Squatiniformes | ||||||
| Family Squatinidae | ||||||
| Squatina californica Ayres, 1859 | O, SD, C | NT | ES | S | D | |
| Subclass Batomorphii |
| Table 1 Continued. | ||||||
| Taxa | ZA | IUCN | NOM | D | S | H |
| Order Torpediniformes | ||||||
| Family Torpedinidae | ||||||
| Torpedo californica Ayres, 1855 | O, SD, C | LC | MA | R | D | |
| Family Narcinidae | ||||||
| Diplobatis ommata (Jordan & Gilbert, 1890) | SD, C, M, P | LC | ES | S | D | |
| Narcine entemedor Jordan & Starks, 1895 | SD, C, M, P | VU | ES | S | D | |
| Order Rhinopristtiformes | ||||||
| Family Rhinobatidae | ||||||
| Pseudobatos glaucostigmus (Jordan & Gilbert, 1883) | SD, C, M, P | VU | ES | S | B | |
| Pseudobatos leucorhynchus Günther, 1867 | SD, C, M, P, G | VU | ES | S | B | |
| Pseudobatos productus (Ayres, 1854) | O, SD, C | NT | ES | S | B | |
| Family Trygonorhinidae | ||||||
| Zapteryx exasperata (Jordan & Gilbert, 1880) | SD, C, M, P | DD | ES | S | D | |
| Order Myliobatiformes | ||||||
| Family Platyrhynidae | ||||||
| Platyrhinoidis triseriata (Jodan & Gilbert, 1880) | SD, C | LC | ES | S | D | |
| Family Urotrygonidae | ||||||
| Urobatis halleri (Cooper, 1863) | SD, C, M, P | LC | ES | S | B | |
| Urobatis maculatus Garman, 1913 | C, M | LC | ES | S | B | |
| Urotrygon aspidura (Jordan & Gilbert, 1882) | SD, M, P | NT | ES | S | B | |
| Urotrygon asterias (Jordan & Gilbert, 1883) | SD, C, M, P | DD | ES | S | B | |
| Urotrygon chilensis (Günther, 1872) | SD, C, M, P | NT | ES | S | B | |
| Urotrygon munda Gill, 1863 | SD, C, M, P | NT | ES | S | B | |
| Urotrygon rogersi (Jordan & Starks, 1895) | SD, C, M, P | NT | ES | S | B | |
| Family Dasyatidae | ||||||
| Hypanus dipterurus (Jordan & Gilbert, 1880) | SD, C, M, P, G | VU | MA | R | D | |
| Hypanus longus (Garman, 1880) | SD, C, M, P, G | VU | MA | R | D | |
| Family Gymnuridae | ||||||
| Gymnura marmorata (Cooper, 1864) | SD, C, M, P | NT | ES | S | D | |
| Family Aetobatidae | ||||||
| Aetobatus laticeps Gill, 1865 | SD, C, M, P | VU | ES | S | D | |
| Family Myliobatidae | ||||||
| Myliobatis californica Gill, 1865 | O, SD, C, M, P | LC | MA | EP | ||
| Myliobatis longirostris Applegate & Fitch, 1964 | SD, C, M, P | VU | ES | S | D | |
| Family Rhinopteridae | ||||||
| Rhinoptera steindachneri Evermann & Jenkins, 1891 | SD, C, M, P, G | NT | MA | S | EP | |
| Class Actinopterygii | ||||||
| Order Elopiformes | ||||||
| Family Elopidae | ||||||
| Elops affinis Regan, 1909 | SD, C, M, P | DD | ES | S | EP | |
| Order Albuliformes | ||||||
| Family Albulidae | ||||||
| Albula gilberti Pfeiler & van der Heiden, 2011 | SD, C | NE | ES | S | D | |
| Order Angulliformes | ||||||
| Family Muraenidae | ||||||
| Echidna nocturna (Cope, 1872) | CT | LC | ES | R | D | |
| Gymnothorax castaneus (Jordan & Gilbert, 1883) | SD, C, M, P, G | LC | ES | R | D | |
| Gymnothorax mordax (Ayres, 1859) | SD, C, M, P, G | LC | MA | R | D | |
| Gymnothorax verrilli (Jordan & Gilbert, 1883) * | SD, C, M, P | LC | ES | S | D | |
| Muraena argus (Steindachner, 1870) | SD, C, M, P, G | LC | MA | R | D | |
| Muraena lentiginosa Jenyns, 1842 * | SD, C, M, P | LC | MA | R | D | |
| Family Ophichthidae | ||||||
| Myrichthys tigrinus Girard, 1859 | SD, C, M, P, G | LC | ES | S | D | |
| Myrophis vafer Jordan & Gilbert, 1883 | SD, C, M, P, G | LC | ES | S | D | |
| Ophichthus triserialis (Kaup, 1856) | O, SD, C, M, P, G | LC | ES | S | D | |
| Ophichthus zophochir Jordan & Gilbert, 1882 | O, SD, C, M, P, G | LC | ES | S | D | |
| Family Congridae | ||||||
| Ariosoma gilberti (Ogilby, 1898) | C, M, P, G | LC | ES | S | B | |
| Gorgasia puntata Meek & Hildebrand, 1923 | SD, C, M, P | LC | ES | S | D | |
| Order Clupeiformes | ||||||
| Family Engraulidae | ||||||
| Anchoa argentivittata (Regan, 1904) | SD, C, M, P | LC | ES | EP | ||
| Anchoa compressa (Girard, 1858) | SD, C | LC | ES | EP | ||
| Anchoa delicatissima (Girard, 1854) | O, SD | LC | ES | EP | ||
| Anchoa exigua (Jordan & Gilbert, 1882) | SD, C, M | LC | ES | EP | ||
| Anchoa helleri (Hubbs, 1921) | SD, C | LC | ES | EP | ||
| Anchoa ischana (Jordan & Gilbert, 1882) | SD, C, M, P | LC | ES | EP | ||
| Anchoa lucida (Jordan & Gilbert, 1882) | SD, C, M | LC | ES | EP | ||
| Anchoa nasus (Kner & Steichnader, 1867) | SD, C, M, P | LC | ES | EP | ||
| Anchovia macrolepidota (Kner, 1863) | SD, C, M, P | LC | ES | EP | ||
| Cetengraulis mysticetus (Günther, 1867) * | SD, C, M, P | LC | ES | EP | ||
| Engraulis mordax Girard, 1854 | SD, C, M, P | DD | ES | EP | ||
| Family Clupeidae | ||||||
| Harengula thrissina (Jordan & Gilbert, 1882) * | S, C, M, P, G | LC | ES | EP | ||
| Lile nigrofasciata Castro-Aguirre, Ruiz-Campos & Balart, 2002 | SD, C, M, P | LC | ES | EP | ||
| Lile stolifera (Jordan & Gilbert, 1882) | SD, C, M, P | LC | ES | EP | ||
| Opisthonema libertate (Günther, 1867) | SD, C, M, P | LC | ES | EP | ||
| Opisthonema medirastre Berry & Barret, 1963 * | SD, C, M, P | LC | ES | EP | ||
| Sardinops sagax (Jenyns, 1842) * | CT | LC | ES | EP | ||
| Family Drussumieriidae | ||||||
| Etrumeus acuminatus Gilbert, 1890 * | SD, C, M, P | LC | MA | EP | ||
| Order Gonorynchiformes | ||||||
| Family Chanidae | ||||||
| Chanos chanos (Forsskål, 1775) | CT | LC | ME | EP | ||
| Order Siluriformes | ||||||
| Family Ariidae | ||||||
| Bagre panamensis (Gill, 1863) | SD, C, M, P | LC | ME | S | EP | |
| Notarius planiceps (Steindachner, 1876) | SD, C, M, P | LC | ME | S | EP | |
| Occidentarius platypogon (Günther, 1864) | SD, C, M, P | LC | ME | S | EP | |
| Order Stomiiformes | ||||||
| Family Phosichthydae | ||||||
| Vicinguerria lucetia (Garman, 1899) * | AP | LC | MA | EP | ||
| Order Aulopiformes | ||||||
| Family Synodontidae | ||||||
| Synodus evermanni Jordan & Bollman, 1890 | SD, C, M, P | LC | ES | S | D | |
| Synodus lacertinus Gilbert, 1890 | SD, C, M, P, G, PC | LC | ES | R | D | |
| Synodus lucioceps (Ayres, 1855) | SD, C, M | LC | ES | S | D | |
| Synodus scituliceps Jordan & Gilbert, 1882 | SD, C, M, P | LC | ES | S | D | |
| Synodus sechurae Hildebrand, 1946 | SD, C, M, P | LC | ES | S | D | |
| Order Myctophiformes | ||||||
| Family Myctophidae | ||||||
| Ceratoscopelus townsendi (Eigenmann & Eigenmann, 1889) * | CT | LC | MA | EP | ||
| Ceratoscopelus warmingii (Lütken, 1892) | CT | LC | MA | EP | ||
| Electrona risso (Cocco, 1829) | CT | LC | MA | EP | ||
| Lampadena urophaos Paxton, 1963 | CT | LC | MA | EP | ||
| Order Gadiformes | ||||||
| Family Merluccidae | ||||||
| Merluccius productus (Ayres, 1853) | SD, C, M | LC | MA | D | ||
| Order Ophidiiformes | ||||||
| Family Ophidiidae | ||||||
| Chilara taylori (Girard, 1858) | SD, C, M, P | LC | ES | S | D | |
| Lepophidium negropinna (Hildebrand & Barton, 1949) * | SD, C, M, P, PC | LC | ES | S | D | |
| Ophidion galeoides (Gilbert, 1890) | SD, C, M, P | LC | ES | S | D | |
| Ophidion scrippsae (Hubbs, 1916) ¨* | SD, C | LC | ES | S | D | |
| Family Dinematichthyidae | ||||||
| Ogilbia ventralis (Gill, 1863) | SD, C, M, P, G | LC | MA | S | D | |
| Order Batrachoidiformes | ||||||
| Family Batrachoididae | ||||||
| Porichthys analis Hubbs & Schultz, 1939 | SD, C, M, P | LC | ES | D | ||
| Porichthys margaritatus (Richardson, 1844) | SD, C, M | LC | ES | D | ||
| Porichthys myriaster Hubbs & Schultz, 1939 | SD, C, M, P | LC | ES | S | D | |
| Porichthys notatus Girard, 1854 | O, SD, C | LC | ES | S | D | |
| Order Lophiiformes | ||||||
| Family Lophiidae | ||||||
| Lophioides caulinaris (Garman, 1899) | SD, C, M, P, G | LC | MA | D | ||
| Lophioides setigerus (Vahl, 1797) | CT | LC | MA | D | ||
| Lophioides spilurus (Garman, 1899) | SD, C, M, P | LC | MA | B | ||
| Family Antennariidae | ||||||
| Fowlerichthys avalonis (Jordan & Starks, 1907) | SD, C, M, P, G | LC | ES | S | D | |
| Family Ogcocephalidae | ||||||
| Zalieutes elater (Jordan & Gilbert, 1882) | SD, C, M, P, G | LC | ES | S | D | |
| Order Mugiliformes | ||||||
| Family Mugilidae | ||||||
| Chaenomugil proboscideus (Günther, 1861) | SD, C, M, P | LC | ES | R | EP | |
| Mugil cephalus Linnaeus, 1758 | CT | LC | ES | S | D | |
| Mugil curema Valenciennes, 1836 | CT | LC | ES | S | D | |
| Mugil hospes Jordan & Culver, 1895 | AA | LC | ES | S | D | |
| Mugil setosus Gilbert, 1892 | SD, C, M, P | LC | MA | D | ||
| Order Atheriniformes | ||||||
| Family Atherinopsidae | ||||||
| Atherinella eriarcha Jordan & Gilbert, 1882* | SD, C, M | LC | ES | EP | ||
| Atherinella nepenthe (Myers & Wade, 1942) * | SD, C, M, P | LC | ES | EP | ||
| Atherinops affinis (Ayres, 1860) * | O, SD, C | LC | ES | EP | ||
| Atherinopsis californiensis Girard, 1854 * | O, SD, C | LC | ES | EP | ||
| Leuresthes tenuis (Ayres, 1860) | O, SD, C | NE | MA | EP | ||
| Order Beloniformes | ||||||
| Family Exocoetidae | ||||||
| Fodiator acutus (Valenciennes, 1847) * | SD, C, M, P | LC | MA | EP | ||
| Prognichthys tringa Breder, 1928 * | SD, C, M, P, G | LC | MA | EP | ||
| Family Hemirhamphidae | ||||||
| Hemirhamphus saltator Gilbert & Starks, 1904 * | O, SD, C, M, P | LC | ES | EP | ||
| Hyporhamphus gilli Meek & Hildebrand, 1923 | SD, C, M, P, G | LC | ES | EP | ||
| Hyporhamphus naos Banford & Collette, 2001 | SD, C, M, P | LC | ES | EP | ||
| Hyporhamphus rosae (Jordan & Gilbert, 1880) * | SD, C, M, P | LC | ES | EP | ||
| Oxyporhamphus micropterus (Valenciennes, 1847) * | CT | NE | MA | EP | ||
| Family Belonidae | ||||||
| Strongylura exilis (Girard, 1854) * | SD, C, M, P | LC | ES | EP | ||
| Order Cyprinodontiformes | ||||||
| Family Fundulidae | ||||||
| Fundulus parvipinnis Girard, 1854 | O, SD | LC | ES | S | D | |
| Order Gasterosteiformes | ||||||
| Family Syngnathidae | ||||||
| Cosmocampus arctus (Jenkins & Evermann, 1889) * | SD, C | LC | ES | R | D | |
| Doryrhamphus excisus Kaup, 1856 | CT | LC | ES | R | D | |
| Hippocampus ingens Girard, 1858 * | SD, C, M, P | VU | Pr | ES | S | D |
| Syngnathus auliscus (Swain, 1882) * | SD, C, M, P | LC | ES | S | D | |
| Syngnathus exillis (Osburn & Nichols, 1916) | O, SD | LC | ES | S | D | |
| Syngnathus leptorhynchus Girard, 1854 * | O, SD | LC | ES | S | D | |
| Family Fistularidae | ||||||
| Fistularia commersonii Rüpell, 1838 | AP | LC | ES | S | D | |
| Fistularia corneta Gilbert & Starks, 1904 | SD, C, M, P, G | LC | ES | S | D | |
| Order Scorpaeniformes | ||||||
| Family Scorpaenidae | ||||||
| Scorpaena guttata Girard, 1854 | SD, C | DD | MA | R | D | |
| Scorpaena mystes Jordan & Starks, 1895 | SD, C, M, P, G | LC | MA | R | D | |
| Scorpaena russula Jordan & Bollman, 1890 | SD, C, M, P | LL | MA | R | B | |
| Scorpaena sonorae Jenkins & Evermann, 1889 | SD, C | LC | MA | R | B | |
| Sebastes auriculatus Girard, 1854 | O, SD | NE | MA | R | B | |
| Sebastes goodei (Eigenmann & Eigenmann, 1890) | O, SD | NE | MA | R | D | |
| Family Triglidae | ||||||
| Bellator gymnostethus (Gilbert, 1892) | SD, C, M, P | LC | MA | B | ||
| Bellator loxias (Jordan, 1897) | SD, C, M, P, G | LC | MA | B | ||
| Bellator xenisma (Jordan & Bollman, 1890) | O, SD, C, M, P | LC | MA | B | ||
| Prionotus birostratus Richardson, 1844 | SD, C, M, P | LC | MA | B | ||
| Prionotus horrens Richardson, 1844 | SD, C, M, P | LC | MA | B | ||
| Prionotus ruscarius Gilbert & Starks, 1904 * | SD, C, M, P | LC | MA | B | ||
| Prionotus stephanophrys Lockington, 1881 | SD, C, M, P | LC | MA | B | ||
| Family Scorpaenichthyidae | ||||||
| Scorpaenichthys marmoratus (Ayres, 1854) * | O, SD | NE | MA | B | ||
| Family Psychrolutidae | ||||||
| Chitonotus pugetensis (Steindachner, 1876) | O, SD | NE | MA | B | ||
| Family Agonidae | ||||||
| Stellerina xyosterna (Jordan & Gilbert, 1880) | O,SD | NE | MA | B | ||
| Order Perciformes | ||||||
| Family Centropomidae | ||||||
| Centropomus medius Günther, 1864 | SD, C, M, P | LC | ES | S | B | |
| Centropomus nigrescens Günther, 1864 | SD, C, M, P | LC | ES | S | B | |
| Family Polyprionidae | ||||||
| Stereolepis gigas Ayres, 1859 | SD, C | CR | ES | S | D | |
| Family Epinephelidae | ||||||
| Cephalopholis panamensis (Steindachner, 1877) | SD, C, M, P | LC | MA | R | D | |
| Dermatolepis dermatolepis (Boulenger, 1895) | SD, C, M, P, G | LC | ES | R | D | |
| Epinephelus analogus Gill, 1863 | SD, C, M, P, G | LC | ES | S | D | |
| Epinephelus labriformis (Jenyns, 1840) | SD, C, M, P, G | LC | MA | R | D | |
| Epinephelus quinquefasciatus (Bocourt,1868) | SD, C, M, P | DD | ES | S | D | |
| Hyportodus niphobles (Gilbert & Starks, 1897) | SD, C, M, P | LC | ES | D | ||
| Mycteroperca jordani (Jenkins & Evermann, 1889) | SD, C, M | EN | ES | D | ||
| Mycteroperca rosacea (Streets, 1877) | SD, C, M | LC | ES | D | ||
| Mycteroperca xenarcha Jordan, 1888 | SD, C, M, P | DD | MA | R | D | |
| Rypticus bicolor Valenciennes, 1846 | SD, C, M, P, G | LC | ES | R | D | |
| Rypticus nigripinnis Gill, 1861 | SD, C, M, P, G | LC | ES | S | D | |
| Family Serranidae | ||||||
| Diplectrum eumelum Rosenblatt & Johnson, 1974 | SD, C, M, P, G | LC | MA | S | D | |
| Diplectrum euryplectrum Jordan & Bollman, 1890 | SD, C, M, P | LC | MA | S | D | |
| Diplectrum labarum Rosenblatt & Johnson, 1974 | SD, C, M, P | LC | MA | S | MP | |
| Diplectrum macropoma (Gpunther, 1864) | SD, C, M, P, G | LC | MA | S | D | |
| Diplectrum maximum Hildebrand, 1946 | O, SD, C, M, P | LC | MA | S | B | |
| Diplectrum pacificum Hildebrand, 1946 | SD, C, M, P | LC | ES | S | D | |
| Diplectrum rostrum Bortone, 1974 | SD, C, M, P, G | LC | MA | S | D | |
| Diplectrum sciurus Gilbert, 1892 | SD, C | LC | MA | S | D | |
| Paralabrax aeroguttatus Waldford, 1936 * | SD, C | DD | MA | R | D | |
| Paralabrax clathratus (Girard, 1854) | O, SD | LC | MA | R | D | |
| Paralabrax maculatofasciatus (Steindachner, 1868) * | SD, C, M, P | LC | MA | S | D | |
| Paralabrax nebulifer (Girard, 1854) * | SD, C, M | LC | MA | R | D | |
| Family Opistognhtidae | ||||||
| Opistognathus punctatus Peters, 1869 | SD, C, M, P, G | LC | MA | S | D | |
| Opistognathus rhomaleus Jordan & Gilbert, 1881 | SD, C, M, P | LC | MA | S | D | |
| Family Priacanthidae | ||||||
| Cookeolus japonicus (Cuvier, 1829) | CT | LC | MA | R | D | |
| Pristigenys serrula (Gilbert, 1891) | SD, C,M, P | LC | MA | R | D | |
| Family Apogonidae | ||||||
| Apogon atricaudus Jordan & McGregor, 1898* | SD, C, M | LC | MA | R | D | |
| Apogon guadalupensis (Osburn & Nichols, 1916) | SD, C, M, G | LC | MA | R | D | |
| Apogon pacificus (Herre, 1935) | SD, C, M, P, G | LC | MA | R | D | |
| Apogon retrosella (Gill, 1862)* | SD, C, M | LC | MA | R | D | |
| Family Malacanthidae | ||||||
| Caulolatilus affinis Gill, 1865 | SD, C, M, P, G | LC | MA | S | D | |
| Caulolatilus princeps (Jenyns, 1840) | O, SD, C, M, P, G | LC | MA | S | D | |
| Family Nematistiidae | ||||||
| Nematistius pectoralis Gill, 1862 | SD, C, M, P | NE | ES | S | D | |
| Family Carangidae | ||||||
| Alectis ciliaris (Bloch, 1787) | CT | LC | ES | S | D | |
| Carangoides otrynter Jordan & Gilbert, 1883 | SD, C, M, P, G | LC | ES | S | D | |
| Carangoides vinctus Jordan & Gilbert, 1882 | SD, C, M, P | LC | MA | S | EP | |
| Caranx caballus Günther, 1868 | SD, C, M, P | LC | ES | S | EP | |
| Chloroscombrus orqueta Jordan & Gilbert, 1883 * | SD, C, M, P | LC | ES | S | D | |
| Decapterus macrosoma Bleeker, 1851 | CT | LC | MA | EP | ||
| Gnathanodon speciosus (Forsskål, 1775) * | CT | LC | ES | S | D | |
| Hemicaranx leucurus (Günther, 1864) | SD, C, M, P | LC | ES | S | D | |
| Hemicaranx zelotes Gilbert, 1898 | O, SD, C, M, P | LC | ES | S | D | |
| Oligoplites altus (Günther, 1868) | SD, C, M, P, PC | LC | ES | S | D | |
| Oligoplites saurus (Bloch & Schneider, 1801) * | SD, C, M, P | LC | ES | S | D | |
| Selene brevoortii (Gill, 1863) | SD, C, M, P | LC | ES | S | D | |
| Selene orstedii Lütken, 1880 | SD, C, M, P, G | LC | ES | S | D | |
| Selene peruviana (Guichenot, 1866) * | SD, C, M, P | LC | ES | S | D, MP | |
| Seriola lalandi Valenciennes, 1833 | CT | LC | ES | S | D, MP | |
| Trachinotus kennedyi Steindachner, 1876 | SD, C, M, P | LC | ES | S | D, MP | |
| Trachinotus paitensis Cuvier, 1832 | SD, C, M, P | LC | ES | S | D, MP | |
| Trachurus symmetricus (Ayres, 1855) | SD, C, M, P | LC | MA | D, MP | ||
| Family Coryphaenidae | ||||||
| Coryphaena hippurus Linnaeus, 1758 | CT | LC | MA | EP | ||
| Family Lutjanidae | ||||||
| Hoplopagrus guentherii Gill, 1862 | SD, C, M, P | LC | ES | S, R | D | |
| Lutjanus aratus (Günther, 1864) | SD, C, M, P | LC | ES | S, R | D | |
| Lutjanus argentiventris (Peters, 1869) * | SD, C, M, P | LC | ES | S, R | D | |
| Lutjanus colorado Jordan & Gilbert, 1882 | SD, C, M, P | LC | ES | S, R | D | |
| Lutjanus guttatus (Steindachner, 1869) | SD, C, M, P | LC | ES | S, R | D | |
| Lutjanus inermis (Peters, 1869) | SD, C, M, P | LC | MA | R | D | |
| Lutjanus jordani (Gilbert, 1898) | SD, C, M, P, G | LC | ES | S, R | D | |
| Lutjanus novemfasciatus Gill, 1862 | SD, C, M, P | LC | ES | S | D | |
| Lutjanus peru (Nichols & Murphy, 1922) | SD, C, M, P, G | LC | MA | S, R | D | |
| Family Lobotidae | ||||||
| Lobotes pacificus Gilbert, 1898 | SD, C, M, P | LC | ES | S | D | |
| Family Gerreidae | ||||||
| Dekertichthys aureolus (Jordan & Gilbert, 1882) | SD, C, M, P | NE | ES | S | D | |
| Diapterus brevirostris (Sauvage, 1879) * | SD, C, M, P | NE | ES | S | D | |
| Eucinostomus currani Zahuranec, 1980 * | SD, C, M, P | LC | ES | S | D | |
| Eucinostomus dowii (Gill, 1863) * | SD, C, M, P | LC | ES | S | D | |
| Eucinostomus entomelas Zahuranec, 1980 | SD, C, M, P | LC | ES | S | D | |
| Eucinostomus gracilis (Gill, 1862) * | SD, C, M, P | LC | ES | S | D | |
| Eugerres lineatus (Humboldt, 1821) | SD, C, M, P | LC | ES | S | D | |
| Gerres cinereus (Walbaum, 1792) | AA | LC | ES | S | D | |
| Family Haemulidae | ||||||
| Anisotremus davidsonii (Steindachner, 1875) | SD, C | LC | MA | R | D | |
| Anisotremus interruptus (Gill, 1862) | SD, C, M, P, G | LC | MA | R | D | |
| Brachygenys californiensis (Steindachner, 1875) | SD, C | LC | MA | R | D | |
| Conodon serrifer Jordan & Gilbert, 1882 | SD, C, M, P | LC | ES | S | D | |
| Haemulon flaviguttatum Gill, 1862 | SD, C, M, P | LC | MA | R | D | |
| Haemulon maculicauda (Gill, 1862) | SD, C, M, P, G | LC | MA | R | D | |
| Haemulon scudderi Gill, 1862 | SD, C, M, P, G | LC | ES | S, R | D | |
| Haemulon sexfasciatum Gill, 1862 | SD, C, M, P | LC | MA | S, R | D | |
| Haemulon steindachneri (Jordan & Gilbert, 1882) | AA | LC | ES | S, R | D | |
| Haemulopsis axillaris (Steindachner, 1869) | SD, C, M, P | LC | ES | S | D | |
| Haemulopsis elongatus (Steindachner, 1879) | SD, C, M, P | LC | ES | S | D | |
| Haemulopsis leuciscus (Günther, 1864) | SD, C, M, P | LC | ES | S | D | |
| Hamulopsis nitidus (Steindachner, 1869) | SD, C, M, P | LC | ES | S | D | |
| Microlepidotus inornatus Gill, 1862 | SD, C, M | LC | ES | S | D | |
| Orthopristis cantharinus (Jenyns, 1840) | SD, C, M, P, G | DD | ES | S | D | |
| Orthopristis chalceus (Günther, 1864) | SD, C, M, P, G | LC | ES | S | D | |
| Orthopristis reddingi Jordan & Richardson, 1895 | SD, C, M, P | LC | ES | S | D | |
| Rhencus macracanthus (Günther, 1864) | SD, C, M, P | LC | ES | S | D | |
| Rhencus panamensis (Steindachner, 1875) | SD, C, M, P | LC | ES | S | D | |
| Rhonciscus bayanus (Jordan & Evermann, 1898) | SD, C, M, P | LC | ES | S | D | |
| Pomadasys branickii (Steindachner, 1879) | SD, C, M, P | LC | ES | S | D | |
| Xenichthys xanti Gill, 1863 | SD, C, M, P | LC | MA | R | D | |
| Family Sparidae Gill 1863 | ||||||
| Calamus brachysomus (Lokington, 1880) * | SD, C, M, P | LC | ES | S | D | |
| Family Polynemidae | ||||||
| Polydactylus approximans (Lay & Bennett, 1839) * | O, SD, C, M, P | LC | ES | S | D | |
| Poldactylus oprecularis (Gill, 1863) | SD, C, M, P, PC | LC | ES | S | D | |
| Family Sciaenidae | ||||||
| Atractoscion nobilis (Ayres, 1860) | O, SD, C | LC | ES | S | D | |
| Bairdiella armata Gill, 1863 | SD, C | LC | ES | S | D | |
| Bairdiella icistia (Jordan & Gilbert, 1882) * | C, M, P, G | LC | ES | S | D | |
| Cheilotrema saturnum (Girard, 1858) | O, SD, C, M, P | DD | MA | R | D | |
| Corvula macrops (Steindachner, 1985) | SD, C, M, P, G | DD | MA | R | D | |
| Cynoscion parvipinnis Ayres, 1861 | O, SD, C | DD | ES | S | D | |
| Cynoscion reticulatus (Günther, 1864) | SD, C, M, P | LC | ES | S | D | |
| Cynoscion squamipinnis (Günther, 1867) | SD, C, M, P | LC | ES | S | D | |
| Cynoscion xanthulus Jordan & Gilbert, 1882 | SD, C, M, P | LC | ES | S | D | |
| Elattarchus archidium (Jordan & Gilbert, 1882) | SD, C, M, P | LC | ES | S | D | |
| Genyonemus lineatus (Ayres, 1855) | O, SD | LC | MA | S | D | |
| Larimus pacificus Jordan & Bollman, 1890 | SD, C, M, P, G, PC | LC | ES | S | D | |
| Menticirrhus elongatus (Günther, 1864) | SD, C, M, P | LC | ES | S | D | |
| Menticirrhus nasus (Günther, 1868) | SD, C, M, P | LC | ES | S | D | |
| Menticirrhus panamensis (Steindachner, 1875) | SD, C, M, P, PC | LC | ES | S | D, MP | |
| Menticirrhus undulatus (Girard, 1854) * | SD, C, M, P | DD | ES | S | D, MP | |
| Micropogonias altipinnis (Günther, 1864) | SD, C, M, P | LC | ES | S | D | |
| Micropogonias ectenes (Jordan & Gilbert, 1882) * | SD, C, M, P | LC | ES | S | D, MP | |
| Ophioscion vermicularis (Günther, 1867) | SD, C, M, P | LC | ES | S | D | |
| Paralonchurus goodei Gilbert, 1898 | SD, C, M, P | LC | ES | S | D | |
| Pareques viola (Gilbert, 1898) | SD, C, M, P | LC | MA | S | D | |
| Roncador stearnsii (Steindachner, 1876) | SD, C, M | LC | ES | S | D | |
| Seriphus politus Ayres, 1860 | O, SD | LC | ES | D, MP | ||
| Stellifer winterteenorum Chao, 2001 | SD, C, M | NE | ES | S | D | |
| Umbrina roncador Jordan & Gilbert, 1882 | C, M | LC | ES | S | D | |
| Umbrina winterteeni Walker & Radford, 1992 | SD, C | DD | ES | S | D | |
| Umbrina xanti Gill, 1862 | SD, C, M, P | LC | ES | S | D | |
| Family Mullidae | ||||||
| Mulloidichthys dentatus (Gill, 1862) | SD, C, M, P | LC | MA | S | D | |
| Pseudupeneus grandisquamis (Gill, 1863) | SD, C, M, P, G | LC | MA | S | D | |
| Family Kyphosidae | ||||||
| Kyphosus azureus (Jenkins & Evermann, 1889) | O, SD, C | LC | ES | R | D | |
| Kyphosus elegans (Peters, 1869) | SD, C, M, P, G | LC | ES | R | D | |
| Kyphosus vaigiensis (Qouy & Gaimard, 1825) | SD, C, M, P, G | LC | MA | R | D | |
| Family Chaetodontidae | ||||||
| Chaetodon humeralis Günther, 1860 | SD, C, M, P, G | LC | MA | R | D | |
| Prognathodes falcifer (Hubbs & Rechnitzer, 1958) | SD, C, M, G | LC | MA | R | D | |
| Family Pomacanthidae | ||||||
| Holocanthus clarionensis Gilbert, 1891 | SD, C, M, P | VU | Pr | MA | S, R | D |
| Holacanthus passer Valenncienes, 1846 | SD, C, M, P | LC | MA | S, R | D | |
| Family Cirrhitidae | ||||||
| Cirrhitus rivulatus Valenciennes, 1846 | SD, C, M, P | LC | MA | D | ||
| Oxyrrhites typus Bleeker, 1857 | CT | LC | MA | R | D | |
| Family Embiotocidae | ||||||
| Embiotoca jacksoni Agazziz, 1853 | SD, C | LC | MA | MP | ||
| Family Pomacentridae | ||||||
| Abudefduf declivifrons (Gill, 1862) | SD, C, M, P | LC | MA | S, R | D | |
| Abudefduf troschelii (Gill, 1862) * | SD, C, M, P | LC | MA | S, R | D | |
| Hypsypops rubicundus (Girard, 1854) * | SD, C, M | LC | MA | R | D | |
| Microspathodon dorsalis (Gill, 1862) | SD, C, M, P, G | LC | MA | R | D | |
| Stegastes acapulcoensis (Fowler, 1944) | SD, C, M, P, G | LC | MA | R | D | |
| Stegastes leucorus (Gilbert, 1892) | SD, C, M, P, G | VU | MA | R | D | |
| Stegastes rectifraenum (Gill, 1862) | SD, C, M | LC | MA | R | D | |
| Stegastes redemptus (Heller & Snodgrass, 1903) | SD | LC | MA | R | D | |
| Family Labridae | ||||||
| Bodianus diplotaenia (Gill, 1862) * | SD, C, M, P, G | LC | MA | R | D | |
| Bodianus pulcher (Ayres, 1854) | O, SD, C | VU | MA | R | D | |
| Halichoeres californicus (Günther, 1861) * | O, SD | LC | MA | S, R | D | |
| Halichoeres chierchiae DiCaporiacco, 1947 | SD, C, M, P | LC | MA | R | D | |
| Halichoeres notospilus (Günther, 1864) | SD, C, M, P, G | LC | ES | S, R | D | |
| Halichoeres semicinctus (Ayres, 1859) | SD, C | LC | MA | R | D | |
| Thalassoma grammaticum Gilbert, 1890 | SD, C, M, P | LC | MA | R | D | |
| Thalassoma lucasanum (Gill, 1862) | SD, C, M, P | LC | MA | S, R | D | |
| Xyrichtys mundiceps Gill, 1862 | SD, C, M | LC | ES | S | D | |
| Family Scaridae | ||||||
| Nicholsina denticulata (Evermann & Radcliffe, 1917) | SD, C, M, P, G | LC | MA | S, R | D | |
| Scarus compressus (Osburn & Nichols, 1916) | SD, C, M, P, G | LC | MA | S, R | D | |
| Scarus ghobban Forsskål, 1775 | CT | LC | MA | S, R | D | |
| Scarus perrico Jordan & Gilbert, 1882 | SD, C, M, P, G | LC | MA | S, R | D | |
| Family Uranoscopidae | ||||||
| Astroscopus zephyreus Gilbert & Starks, 1897 | SD, C, M, P | LC | MA | S | D | |
| Kathetostoma averruncus Jordan & Bollman, 1890 | O, SD, C, M, P, G | LC | MA | S | D | |
| Family Tripterygiidae | ||||||
| Axoclinus storeyae (Brock, 1940) | SD, C | LC | MA | S, R | D | |
| Enneanectes carminalis (Jordan & Gilbert, 1882) * | SD, C, M, P | LC | MA | R | D | |
| Family Dactyloscopidae | ||||||
| Dactylagnus mundus Gill, 1862 | SD, C, M, P, G | LC | MA | S | D | |
| Dactyloscopus elongatus Myers & Wade, 1946 | SD, C, M | LC | MAR | S | D | |
| Dactyloscopus pectoralis Gill, 1861 | SD, C, M | NE | MA | S | D | |
| Gillellus semicinctus Gilbert, 1890 | SD, C, M, P, G | LC | MAR | S | D | |
| Heteristius cinctus (Osburn & Nichols, 1916) | SD, C, M, P | LC | MAR | S | D | |
| Family Blennidae | ||||||
| Hypsoblennius brevipinnis (Günther, 1861) | SD, C, M, P, G | LC | MAR | R | B | |
| Hypsoblennius gentilis (Girard, 1840) * | SD, C | LC | ES | R | B | |
| Hypsoblennius gilberti (Jordan, 1882) * | O, SD | LC | MAR | R | D | |
| Hypsoblennius jenkinsi (Jordan & Evermann, 1896) * | SD, C | LC | MA | R | D | |
| Ophioblennius steindachneri Jordan & Evermann, 1898 * | SD, C, M, P, G | LC | MAR | R | D | |
| Plagiotremus azaleus (Jordan & Bollman, 1890) | SD, C, M, P, G | LC | MAR | R | D | |
| Family Clinidae | ||||||
| Gibbonsia elegans (Cooper, 1864) | O, SD, C | LC | MA | S | B | |
| Family Labrisomidae | ||||||
| Labrisomus multiporosus Hubb, 1953 * | SD, C, M, P, G | LC | MA | R | B | |
| Labrisomus xanti Gill, 1860* | SD, C, M, G | LC | MA | R | B | |
| Malacoctenus hubbsi Springer, 1959 * | SD, C | LC | MA | R | B | |
| Malacoctenus tetranemus (Cope, 1877) | SD, C, M, P, G | LC | MA | R | B | |
| Paraclinus asper (Jenkins & Evermann, 1889) * | SD, C | LC | MA | R | B | |
| Paraclinus integripinnis (Smith, 1880) * | SD, C, M | LC | MA | R | B | |
| Paraclinus magdalenae Rosenblatt & Parr, 1969 | SD, E | EN | MA | R | B | |
| Paraclinus sini Hubbs, 1952 | SD, C | LC | MA | R | B | |
| Paraclinus stephensi Rosenblatt & Parr, 1969 | SD, M | LC | MA | R | B | |
| Family Chaenopsidae | ||||||
| Chaenopsis alepidota (Gilbert, 1890) * | SD, C, M, P | LC | MA | S | B | |
| Cirriemblemaria lucasana (Stephens, 1963) | SD, C, M | LC | MA | R | B | |
| Coralliozetus micropes (Beebe & Tee-Van, 1938) | SD, C | LC | MA | R | B | |
| Coralliozetus rosenblatti Stephens, 1963 | SD, C | LC | MA | R | B | |
| Protemblemaris bicirrus (Hildebrand, 1946) | SD, C, M, P | LC | MA | R | B | |
| Stathmonotus sinuscalifornici (Chabanaud, 1842) * | SD, C | LC | MA | R | B | |
| Family Gobioesocidae | ||||||
| Gobiesox papillifer Gilbert, 1890 | SD, C, M, P | LC | MA | R | B | |
| Rimicola eigenmanni (Gilbert, 1890) | SD | NE | MA | R | B | |
| Tomicodon zebra (Jordan & Gilbert, 1882) | SD, C, M, P | LC | MA | R | B | |
| Family Callionymidae | ||||||
| Synchiropus atrilabiatus (Garmann, 1899) | SD, C, M, P, G | LC | MA | S | D | |
| Family Eleotridae | ||||||
| Gobiomorus maculatus (Günther, 1859) | SD, C, M, P, G | LC | ES | S | D | |
| Family Gobiidae | ||||||
| Bathygobius ramosus Ginsburg, 1947 | SD, C, M, P | LC | ES | S | B | |
| Ctenogobius sagittula (Günther, 1861) | SD, C, M, P | LC | ES | S | B | |
| Coryphopterus urospilus Ginsburg, 1938 | SD, C, M, P, G | LC | MA | R | B | |
| Gillichthys mirabilis (Cooper, 1864) * | O, SD, C | LC | ES | S | B | |
| Gobulus crescentalis (Gilbert, 1892) | SD, C, M, P | LC | MA | R | B | |
| Gymneleotris seminuda (Günther, 1864) | SD, C, M, P | LC | MA | R | B | |
| Ilypnus gilberti (Eigenmann & Eigenmann, 1889) * | O, SD | LC | ES | S | B | |
| Lythrypnus dalli (Gilbert, 1890) * | SD, C, M, P, G | LC | MA | R | B | |
| Lythrypnus pulchellus Ginsburg, 1938 | SD, C, M, P | LC | MA | R | B | |
| Lythrypnus zebra (Gilbert, 1890) * | O, SD, C | LC | MA | R | B | |
| Microgobius erectus Ginsburg, 1938 | SD, C, M, P | LC | ES | S | B | |
| Microgobius miraflorensis Gilbert & Starks, 1904 | SD, C, M, P | LC | ES | S | B | |
| Microgobius tabogensis Meek & Hildebrand, 1928 | SD, C, M, P | LC | ES | S | B | |
| Quietula y-cauda (Jenkins & Evermann, 1889) * | SD, C | LC | ES | S | B | |
| Typlogobius californiensis Steindachner, 1879 | SD | NE | MA | R | B | |
| Family Microdesmidae | ||||||
| Microdesmus dorsipunctatus Dawson, 1968 | SD, C, M, P | DD | ES | S | B | |
| Family Ephippidae | ||||||
| Chaetodipterus zonatus (Girard, 1858) * | SD, C, M, P | LC | ES | S | D | |
| Family Zanclidae | ||||||
| Zanclus cornutus (Linnaeus, 1758) | CT | NE | MA | R | D | |
| Famila Acanthuridae | ||||||
| Prionotus laticlavius (Valenncienes, 1846) | SD, C, M, P | LC | MA | R | D | |
| Family Sphyraenidae | ||||||
| Sphyraena argentea Girard, 1854 | O, SD, C | LC | MA | S | D | |
| Sphyraena ensis Jordan & Gilbert, 1882 * | SD, C, M, P. PC | LC | MA | S | D | |
| Sphyraena lucasana Gill, 1863 | SD, C, M, P | DD | MA | S | D | |
| Family Scombridae | ||||||
| Auxis thazard (Lacepède, 1800) | CT | LC | MA | D | ||
| Euthynnus affinis (Cantor, 1849) | CT | LC | MA | D | ||
| Sarda chiliensis (Cuvier, 1832) | O, SD, C, M, P, PC | LC | MA | D | ||
| Scomber japonicus Houttuyn, 1782 | CT | LC | MA | D | ||
| Scomberomorus sierra Jordan & Starks, 1895 * | SD, C, M, P | LC | MA | D | ||
| Thunnus obesus (Lowe, 1839) | CT | VU | MA | D | ||
| Famillia Stromateidae | ||||||
| Peprilus simillimus (Ayres, 1860) | O, SD, C | LC | MA | D | ||
| Order Pleuronectiformes | ||||||
| Famila Paralichthydae | ||||||
| Acylopsetta dendritica Gilbert, 1890 | SD, C, M, P | LC | ES | S | B | |
| Citharichthys fragilis Gilbert, 1890 | SD, C | LC | ES | S | B | |
| Citharichthys gilberti Jenkins & Evermann, 1889 | SD, C, M, P | LC | ES | S | B | |
| Citharichthys sordidus (Girard, 185) | O, SD | LC | ES | S | B | |
| Citharichthys stigmaeus Jordan & Gilbert, 1882 | O, SD | LC | ES | S | B | |
| Citharichthys xanthostigma Gilbert, 1890 | SD | LC | ES | S | B | |
| Cyclopsetta panamensis (Steindachner, 1876) | SD, C, M, P | LC | ES | S | B | |
| Etropus crossotus Jordan & Gilbert, 1882 * | SD, C, M, P | LC | ES | S | B | |
| Etropus peruvianus Hildebrand, 1946 | SD, C, M, P | LC | ES | S | B | |
| Hippoglossina bollmani Gilbert, 1890 | SD, C, M, P, G | LC | ES | S | B | |
| Hippoglossina tetrophthalma (Gilbert, 1890) | SD, C, M, P | LC | MA | S | B | |
| Paralichthys aestuarius Gilbert & Scofield, 1898 | SD, C, M | DD | ES | S | B | |
| Paralichthys californicus (Ayres, 1859) * | SD, C | LC | ES | S | B | |
| Paralichthys woolmani Jordan & Williams, 1897 | SD, C, M, P, G | DD | ES | S | B | |
| Syacium latifrons (Jordan & Gilbert, 1882) | SD, C, M, P, G | LC | ES | S | B | |
| Syacium ovale (Günther, 1864) | SD, C, M, P | LC | ES | S | B | |
| Xystreurys liolepis Jordan & Gilbert, 1880 * | SD, C | LC | ES | S | D | |
| Family Pleuronectidae | ||||||
| Pleuronichthys coenosus Girard, 1854 | O, SD | LC | MAS | R | B | |
| Pleuronichthys guttulatus Girard, 1854 | O, SD, C | LC | ES | S | B | |
| Pleuronichthys ocellatus Starks & Thomson, 1910 | SD, C | LC | ES | S | B | |
| Pleuronichthys ritteri Starks & Morris, 1907 | O, SD | LC | ES | S | B | |
| Pleuronichthys verticalis Jordan & Gilbert, 1880 | O, SD, C | LC | ES | S | B | |
| Family Achiridae | ||||||
| Achirus mazatlanus * | SD, C, M, P | LC | ES | S | B | |
| Family Bothidade | ||||||
| Bothus constellatus (Jordan, 1889) | AA | LC | ES | S | B | |
| Bothus leopardinus (Günther, 1862) | SD, C, M, P | LC | ES | S | B | |
| Monolene dubiosa Garman, 1899 | SD, M, P | LC | ES | S | B | |
| Family Cynoglossidae | ||||||
| Symphurus atramentatus Jorsan & Bollman, 1890 | SD, C, M, P | LC | ES | S | B | |
| Symphurus atricaudus (Jordan & Gilbert, 1880) | SD | LC | ES | S | B | |
| Symphurus fasciolaris Gilbert, 1892 | SD, C, M, P | LC | ES | S | B | |
| Symphurus williamsi Jordan & Culver, 1895 | SD, C, M, P | LC | ES | S | B | |
| Order Tetraodonfiformes | ||||||
| Family Balistidae | ||||||
| Balistes polylepis Steindachner, 1876 * | SD, C, M, P | LC | MA | R | D | |
| Pseudobalistes naufragium (Jordan & Starks, 1895) | SD, C, M, P, G | LC | MA | R | D | |
| Suflamen verres (Gilbert & Starks, 1904) | SD, C, M, P, G | LC | MA | R | D | |
| Family Monacanthidae | ||||||
| Aluterus monoceros (Linnaeus, 1758) | CT | NE | MA | S, R | MP | |
| Family Tetraodontidae | ||||||
| Sphoeroides annulatus (Jenyns, 1842) | SD, C, M, P | LC | ES | S | D | |
| Sphoeroides angusticeps (Jenyns, 1842) | SD, C, M, P, G | LC | ES | S | D | |
| Sphoeroides lispus Walker, 1996 | SD, C | LC | ES | S | D | |
| Sphoeroides lobatus (Steindachner, 1870) * | SD, C, M, P, G | LC | ES | S | D | |
| Sphoeroides sechurae Hildebrand, 1946 | SD, C, M, P | LC | ES | S | D | |
| Family Diodontidae | ||||||
| Chilomycterus reticulatus (Linnaeus, 1758) | CT | LC | ES | S | D | |
| Diodon holocanthus Linnaeus, 1758 | CT | LC | ES | S | D | |
| Diodon hystrix Linnaeus, 1758 | CT | LC | ES | S | D |
Ecologically, 56.1% (233 species) are marine-euryhaline, and 42.6% (177 species) of marine-stenohaline derivation. Most of the fish species inhabit soft (235 species = 57%) or rocky (114 species = 27.5%) bottoms. Based on its distribution in the water column,14.4% (60 species) are pelagic, 25% (102 species) benthic and 60% (248 species) demersal; likewise, 262 species (63.1%) were classified as neritic, 55 (13.3%) as epipelagic, 26 (13.2%) as mesopelagic, 109 (26.3%) as benthopelagic, and 7 (1.7%) as bathypelagic.
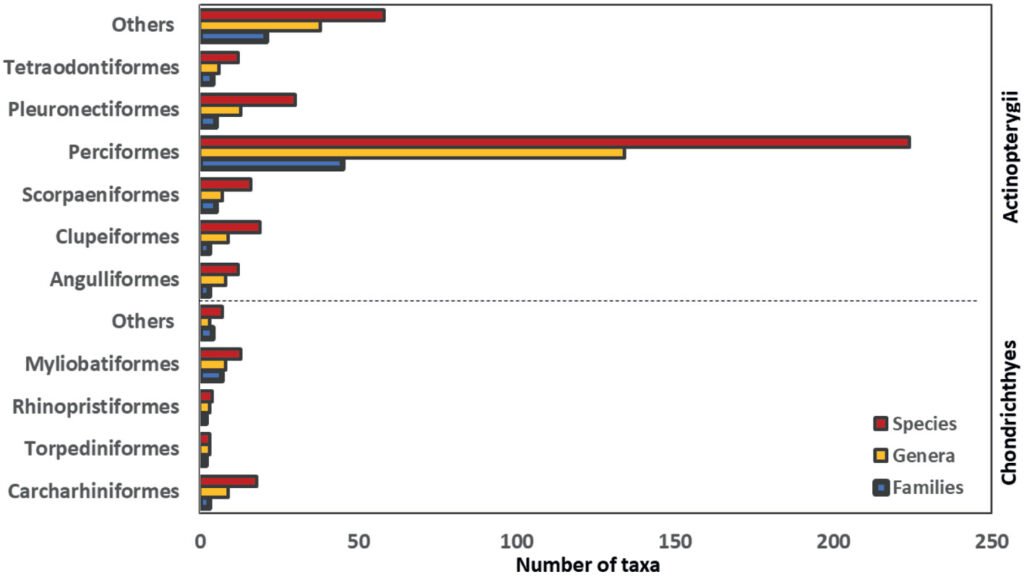
Figure 2. Taxa (families, genera, and species) reported here for the BMA lagoon system.
Discussion
The BMA lagoon system is in a temperate-tropical transition zone where abrupt faunal changes occur (Castro-Aguirre et al., 1992, 1993; Hubbs, 1960; Ruiz-Campos et al., 2010), promoting the existence of a great variety of fish species ranging between 62 (Rodríguez-Romero et al., 2012) to 302 (Galván-Magaña et al., 2000), contrasting with the 416 fish species reported in this study (Table 1, Fig. 2). The great fish diversity found in this marine-estuarine ecosystem results from the coexistence of 3 faunal assemblages of temperate and tropical-subtropical (eurythermal species) derivation, which settled in this area by ancient invasive and colonization migrations (north-south and vice versa) related to geological and oceanographic episodes occurred between Miocene and Pliocene, during the evolution of the BCP and the Gulf of California (Bennett et al., 2015; Castro-Aguirre & Torres-Orozco, 1993; Ferrari et al., 2013).
Differences between the fish species richness previously reported and the one reported here (Fig. 3), result from the increase of ichthyological studies carried out in the area as well as the addition of updated literature records that include range expansion of some fish species like the Clarion angelfish H. clarionensis (Pomacanthidae) and 2 members of the family (Pomacentridae): the Acapulco damselfish Stegastes acapulcoensis (Fowler, 1944) and the Whitetail damselfish Stegastes leucorus (Gilbert, 1892). These tropical fish species reach their northernmost distribution ranges in temperate environments of the northeastern Pacific, due to the warm water intrusion into the temperate region provoked by positive temperature anomalies occurred during 2014 (Hernández-Velasco et al., 2016). Likewise, it has been established as a potential cause the ability of some species to capitalize physiological (e.g., oxygen requirements) and ecological adaptations (e.g., food availability and suitable substrata), regardless of the latitudinal limit of its distribution (Hastings et al., 2020). Other fish species recently included in the checklist of the BMA lagoon system (Love et al. 2021) are the Gulf anchovy Anchoa helleri (Hubbs, 1921) which was reported as endemic of the Gulf of California by González-Acosta, Balart et al. (2018) and the Clarion damselfish, Stegastes redemptus (Heller & Snodgrass, 1903) caught at Hughes Point East of Bahía Santa María (GBIF, 2023).
The checklist reported here includes 76 fish species in the larval stage which also are represented as juveniles and/or adults and contrast with the larval richness (105 to 120 species) previously reported by Avendaño-Ibarra et al. (2004) and Funes-Rodríguez et al. (2007). However, only 12.5% (n = 9) of the fish species listed here have been reported in larval stage, the cardinal fishes (Apogonidae): Apogon atricaudus Jordan & McGregor, 1898 and Apogon retrosella (Gill, 1862); the garibaldi (Pomacanthidae): Hypsypops rubicundus (Girard, 1864); the Mexican hogfish (Labridae): Bodianus diplotaenia (Gill, 1862); the delicate tiplefin (Tripterigiidae): Enneanectes carminalis (Jordan & Gilbert, 1882); the labrisomid blennies: Labrisomus xanti Gill, 1860 and Paraclinus integripinnis (Smith, 1880); and the tube blennies (Chaenopsidae): Chaenopsis alepidota (Gilbert, 1890) and Stathmonotus sinuscalifornici (Chabanaud, 1942). Other 4 taxa not listed here have been recorded at generic level: Melamphaes Günther, 1864 (Malamphaidae), Myripristis Cuvier, 1829 (Holocentridae), Serranus Cuvier, 1816 (Serranidae), and Chromis Cuvier, 1814 (Pomacentridae), as part of the fish fauna of BMA lagoon system (e.g., Avendaño-Ibarra et al. 2004). Thus, once these taxa can be identified to the species level, the fish diversity of this ecosystem could continue to increase.
As previously reported for different lagoon systems along the Mexican Pacific basin (Castro-Aguirre et al., 2006; Gómez-González et al., 2012; González-Acosta, Balart et al., 2018; González-Acosta, Monsalvo-Flores et al., 2021; González-Acosta, Rodiles-Hernández et al., 2018), the high diversity of actinopterygian fishes (371 species) over chondrichthyans (45 species) is remarkable in the BMA lagoon system (Fig. 2). The limited presence of cartilaginous fish in this coastal biotope could be mainly due to the reduced size of the tidal-channel inlets, as well as the poverty of suitable habitats for large-sized sharks and rays (González-Acosta, Balart et al., 2018; González-Acosta, Rabadán-Sotelo et al., 2015; González-Acosta, Rodiles-Hernández et al., 2018; González-Acosta, Ruiz-Campos et al., 2015), in addition to its limited tolerance to wide variations in salinity as is characteristic in coastal ecosystems of arid zones (González-Acosta, Ruiz-Campos et al., 2021). However, it has been reported that along the BCP there are coastal areas that favor their presence as well as the reproduction, breeding and feeding activities of some elasmobranchs. Therefore, the low number of chondrichthyans reported for BMA lagoon system also could be consequence of the discarding of species lacking commercial value during artisanal fishing activities or due to the need to make a greater effort focused on the capture and registration of these fishes that inhabit this ecosystem, as it has been reported in other coastal lagoons of the BCP (González-Acosta, Balart et al., 2018).
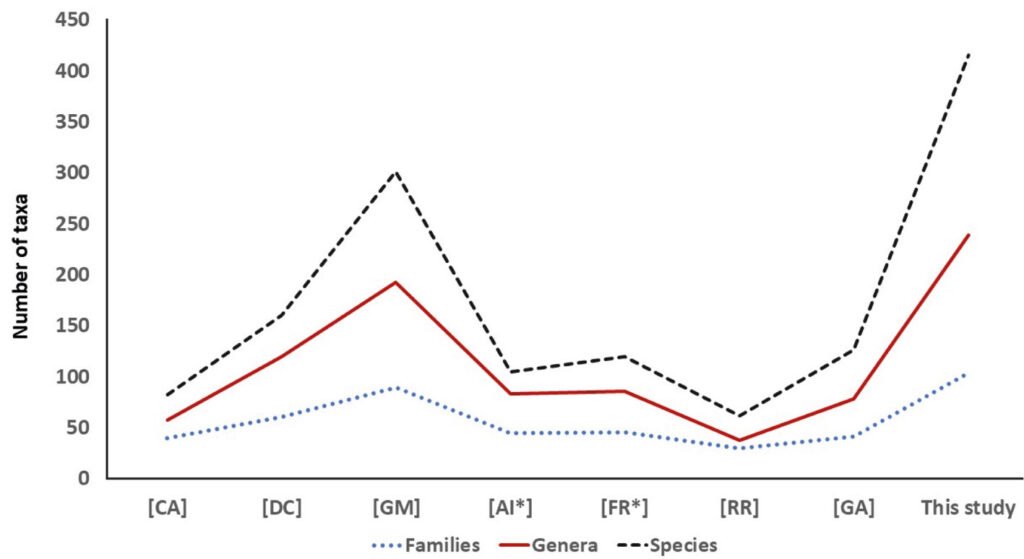
Figure 3. Number of families, genera, and species from the BMA lagoon system, according to different checklist and current data. In square brackets is indicated the corresponding reference: [CA] Castro-Aguirre and Torres-Orozco (1993), [DC] De la Cruz-Agüero et al. (1994), [GM] Galván-Magaña et al. (2000), [AI] Avendaño-Ibarra et al. (2004), [FR] Funes-Rodríguez et al. (2007), [RR] Rodríguez-Romero et al. (2012), and [GA] González-Acosta, Ruiz-Campos et al. (2015).
The highest species richness in the family Carcharhinidae and the genus Carcharhinus in costal ecosystems has been related to their wide distribution in oceans worldwide (Dulvy et al., 2008; Lea & Rosenblatt, 2000). While the higher richness of rays and skate species in the family Urotrygonidae and the genus Urotrygon Gill, 1863, could be associated with their preference for shallow areas with sandy bottoms and abundant vegetation (González-Acosta, Balart et al., 2018).
Otherwise, the high diversity of ray-finned fish species (Actinopterygii) in the BMA lagoon system, is due to the great availability of suitable soft-bottom and submerged vegetation habitats (e.g., mangrove biotopes), as well as favorable hydrological conditions for a variety of actinopterygian fish species, as is common pattern along the subtropical and tropical coastal ecosystems from the northeastern and the Central Mexican Pacific regions (Gómez-González et al., 2012; González-Acosta, Balart et al., 2018; González-Acosta, Rodiles-Hernández et al., 2018; González-Acosta, Ruiz-Campos et al., 2015; Tapia-García et al., 1998). Particularly, for those fish of the order Perciformes and their representative families and species (e.g., Sciaenidae, Haemulidae, Carangidae and Gobiidae) which regularly enter to the coastal ecosystems for different activities (e.g., spawning, nursery and feeding) due to their different life cycles (González-Acosta, Balart et al., 2018; González-Acosta, Ruiz-Campos et al., 2015, 2021).
Moreover, the high proportions (> 80%) in the zoogeographic affinity of the fish fauna from BMA lagoon system, between the California (San Diego and Cortez provinces) and Tropical Eastern Pacific (Mexican and Panamian provinces) regions, corroborate the recognition of this area as a transition zone where coexist fish assemblages from temperate and tropical-subtropical derivation (Briggs, 1974; Castro-Aguirre et al., 1993; González-Acosta, Balart et al., 2018; González-Acosta, Ruiz-Campos et al., 2015; Hastings et al., 2010; Hubbs, 1960; Ruiz-Campos et al., 2010), as well as the ancient relationship between 2 of the main biogeographical regions of the Eastern Pacific (Brusca et al., 2005; Hastings, 2000; Hastings et al., 2010). Particularly, for those fish species whose distribution ranges include boreal-temperate and subtropical-tropical latitudes.
Based on the patterns of distribution of the BMA fish fauna, the status of the endemic Magdalena blenny P. magdalenae was determined. This labrisomid fish species, only known from Magdalena and Santa María bays, Margarita Island and South of Punta Redonda, BCS (GBIF, 2023; Love et al., 2021; Rosenblatt & Parr, 1969), inhabits rocky bottoms at 8 to 21m depths (Robertson & Allen, 2015); however, there is no more information on this species.
Comparatively, the BMA lagoon system hosts a smaller number of marine fish species with circumtropical distribution than other coastal environments of the BCP (González-Acosta, Balart et al., 2018), due to the greater influence of the boreal-temperate region and the California Current over the tropical biogeographic region. However, the presence of fish assemblages with circumtropical affinity such as sharks: mackerel sharks (1 species), requiem sharks (5 species) and hammerheads (3 species), and actinopterygian fishes (e.g., milkfishes, lanternfishes, jacks, dolphinfishes, parrotfishes, Morish idols, mackerels and porcupinefishes) could be since these groups can reach high latitudes during warm ocean conditions in the North Pacific provoked by El Niño and post-El Niño events, as well as their condition as free-swimming fishes (e.g., sharks and jacks), and ontogenetic adaptation (e.g., parrotfishes and Morish idols) during these warm-water events (Lea & Rosenblatt, 2000). The recent range extension records of the Clarion angelfish (Pomacanthidae) and Acapulco damselfish and the whitetail damselfish (Pomacentridae) occurred during the 2014 warm-water period in the BMA lagoon system (Hernández-Velasco et al., 2016) confirm the tendency of these tropical fish families to extend their distribution ranges beyond their northern limits in the Tropical Eastern Pacific as had been documented previously by Lea and Rosenblatt (2000) for other representative species of these fish families.
The presence (although in a lower percentage) of species with amphipacific (e.g., the Pacific spiny dogfish and the reef cornetfish) and amphiamerican (e.g., the lemon shark, the bonnethead shark, the hospe mullet and the Latin grunt) distribution, corroborates the ancient connection between the Tropical Eastern Pacific with the Indo-Pacific and the Western Atlantic biogeographic regions; may be as result of different geologic and oceanographic events which occurred during the evolution of these oceanic basins (González-Acosta, Monsalvo-Flores et al., 2021).
The conservation status (sensu IUCN, 2022) of the fish fauna of the BMA lagoon system corresponds mainly to “Least Concern” species (82%), followed by “Data Deficient” (5%), “Not Evaluated” (4.1%7) and “Near Threatened” (2.4%), which include mainly bony fishes (except for the last category that includes shark and rays). The high number of fish species here reported as “Least Concern” and “Data Deficient” is similar to those reported in different coastal systems around the BCP (González-Acosta, Balart et al., 2018; González-Acosta, Ruiz-Campos et al., 2015, 2021) and the Central Mexican Pacific (González-Acosta, Rodiles-Hernández et al., 2018); these categories do not mean low risk but indicate the lack of complementary information on the basic biology for many fish species. Contrasting with 2 hammerhead shark species (S. lewini and S. mokarran) included as “Critically Endangered” which are widely distributed in warm waters of the World Ocean (GIBF, 2023) and commonly threatened due to targeted or incidental overfishing (Dulvy et al., 2008; González-Acosta, Monsalvo-Flores et al., 2021); and the actinopterygian S. gigas distributed in the Northeastern Pacific but without any ecological or commercial interest in the area (Cornish, 2004; González-Acosta, Balart et al., 2018). Nineteen species (including sharks, rays, and bony fish species) are listed as “Vulnerable” (sensu IUCN,2022), most corresponds to elasmobranchs which were updated in their risk category status in the version 2021-1 of the IUCN Red List (González-Acosta, Monsalvo-Flores et al., 2021); whereas some bony fishes included in this risk category such as H. ingens (Syngnathidae), H. clarionensis (Pomacanthidae) and S. leucorus (Pomacentridae), are commercially exploited by ornamental fishery in Mexico, as well as potentially affected by perturbation of their preferential habitat. A similar treatment was applied to shark species listed as “Endangered”, while some bony fishes such as Mycteroperca jordani (Jenkins & Evermann, 1889) which is caught by artisanal fishery and P. magdalenae recognized as endemic of this region, are included in this risk category under such criteria.
Moreover, the Clarion angelfish (H. clarionensis) and the Pacific seahorse (H. ingens) are included as species with a degree of vulnerability by the NOM-059-SEMARNAT-2010 (DOF, 2010, 2019) under the category of “Special Protection (Pr)”, coinciding with the IUCN Red Lists (IUCN, 2022) where both fish species are listed as “Vulnerable”; however, beyond its commercial importance and limited distribution there is no complementary information that allows corroborating its population status as well as its inclusion in this risk category.
The predominance of marine (stenohaline) and estuarine (euryhaline) fish assemblages in the BMA lagoon system, able to tolerate a wide range of salinities is mainly due to the absence of rain and river runoffs that together with high evaporation rates (that increase the salinity in the water column) make of this ecosystem a negative estuary throughout the year (Álvarez-Borrego et al., 1975; Castro-Aguirre & Torres-Orozco, 1993), as is common in this arid region (Brusca et al., 2005; González-Acosta, Monsalvo-Flores et al., 2021; González-Acosta, Ruiz-Campos et al., 2015); therefore, limiting the presence of fishes from primary and secondary freshwater derivation.
The analysis of distribution by habitat (substrata), allowed to determine that the soft (57%) and rocky (27%) bottoms are the preferential habitats of most fishes of BMA lagoon system, which make use of intertidal areas (including rocky reefs) and the mangrove biotopes associated to this ecosystem as nursery, protection and feeding grounds during their life cycle (larvae, juvenile and adult), alternating seasonally their abundances and generating critical links in the food chains between the lagoon system and the adjacent sea (González-Acosta, Balart et al., 2018; González-Acosta, Ruiz-Campos et al., 2015). Thereby, this habitat contributes to the recruitment of commercial fish species, as well as for those subject to ecological conservation in this region (Avendaño-Ibarra et al., 2004; Cota-Nieto et al., 2015; Hastings & Fischer, 2001).
Ecologically, most of the fish species in the BMA lagoon system exhibit benthic-demersal (84.3%) as well as neritic (63%) habits as correspond to a shallow coastal ecosystem or due to the selectivity of the sampling method commonly used to catch the fish fauna. Also notable is the importance of the BMA lagoon system as nursery and recruitment ground for mesopelagic, benthopelagic and bathypelagic fish species (some of them in larval stages), supporting the linkage between the coastal zone with the adjacent sea. Therefore, their presence indicates the migration process that several species carry out from the open sea to the interior (and vice versa) of the BMA lagoon system influenced by tidal currents.
Because the BMA lagoon system is considered as a priority natural area subject to conservation, the updated checklist here reported, including notes on taxonomy, zoogeography, and conservation status, could contribute to improving our knowledge on the fish resources inhabiting this coastal ecosystem and for the design and implementation of effective fishing regulation strategies, and conservation programs for fish species inhabiting this coastal ecosystem.
Acknowledgements
This study was partially supported by the Instituto Politécnico Nacional (Projects SIP-IPN 20220356, 20231049); IMIPAS-CRIAP, La Paz (BCS) (Pacific Shrimp Program 20142020); and Fishery Improvement Project (FIP 7142). AFGA thanks to EDI and COFAA-IPN Programs. AFGA, CHRQ, and GRC thank the support of SNI Conahcyt Program. Karen Link´s Editorial Services edited the English manuscript. We dedicate this contribution to the memory of JFL, who passed away during the editorial process.
References
Acosta-Velázquez, J., & Ruiz-Luna, A. (2007). Variación en la cobertura, distribución y estructura de los manglares del complejo lagunar Bahía Magdalena-Bahía Almejas (1990-2005). In R. Funes-Rodríguez, J. Gómez-Gutiérrez, & R. Palomares-García (Eds.), Estudios ecológicos en Bahía Magdalena (pp. 127–141). México D.F.: CICIMAR-IPN.
Álvarez-Borrego, S., Galindo-Beet, A., & Chee-Barragan, C. (1975). Características hidroquímicas de Bahía Magdalena, B.C.S. Ciencias Marinas, 2, 94–109. https://doi.org/10.7773/cm.v.212.285
Allen, G. R., & Robertson, D. R. (1994). Fishes of the tropical eastern Pacific. Bathurst, Hawaii: Crawford House Press.
Avendaño-Ibarra, R., Funes-Rodríguez, R., Hinojosa-Medina, A., González-Armas, R., & Aceves-Medina, G. (2004). Seasonal abundance of fish larvae in a subtropical lagoon in the west coast of the Baja California Peninsula. Estuarine Coastal and Shelf Science, 61, 125–135. https://doi.org/10.1016/j.ecss.2004.03.017
Bennett, S. E. K., Oskin, M. E., Vorsey, R. J., Irondo, A., & Kunk, M. J. (2015). Stratigraphy and structural development of the southwest Isla Tiburon marine basin: implications for latest Miocene tectonic opening and flooding of the northern Gulf of California. Geosphere, 11, 977–1007. https://doi.org/10.1130/GES01153.1
Briggs, J. C. (1974). Marine zoogeography. New York: McGraw-Hill.
Brusca, R. C., Findley, L. T., Hastings, P. A., Hendricks, M. E., Torre-Cosio, J., & van der Heiden, A. M. (2005). A. Macrofaunal diversity in the Gulf of California. In J. L. Cartron, G. Ceballos, & R. S. Felger (Eds.), Biodiversity, ecosystems, and conservation in northern Mexico. Madison, New York: Oxford University Press Inc.
Castro-Aguirre, J. L., & Torres-Orozco, R. (1993). Consideraciones acerca del origen de la ictiofauna de Bahía Magdalena-Almejas, un sistema lagunar de la costa occidental de Baja California Sur, México. Anales de la Escuela Nacional de Ciencias Biológicas, 38, 67–73.
Castro-Aguirre, J. L., & Espinosa-Pérez, H. (2006). Los peces de la familia Atherinopsidae (Teleostei: Atheriniformes) de las lagunas neutras e hipersalinas de México. Hidrobiológica, 16, 89–101.
Castro-Aguirre, J. L., Ramírez-Ortiz, J. C., & Martínez-Muñoz, M. A. (1992). Nuevos datos sobre la distribución de lenguados (Pisces: Pleuronectiformes) en la costa del oeste de Baja California, México, con aspectos biológicos y zoogeográficos. Anales de la Escuela Nacional de Ciencias Biológicas, 37, 97–119.
Castro-Aguirre, J. L., Schmitter-Soto, J. J., Balart, E. F., & Torres-Orozco, R. (1993). Sobre la distribución geográfica de algunos peces bentónicos de la costa oeste de Baja California Sur, México, con consideraciones ecológicas y evolutivas. Anales de la Escuela Nacional de Ciencias Biológicas, 38, 75–102.
Castro-Aguirre, J. L., Espinosa-Pérez, H., & Schmitter-Soto, J. J. (1999). Ictiofauna estuarino-lagunar y vicaria de México. México D.F.: Limusa-Noriega.
Castro-Aguirre, J. L., González-Acosta, A. F., & De la Cruz-Agüero, J. (2005). Lista anotada de las especies ícticas anfipacíficas, de afinidad boreal, endémicas y anfipeninsulares del Golfo de California, México. Revista Universidad y Ciencia, 21, 85–106. https://doi.org/10.19136/era.a21n42.335
Castro-Aguirre, J. L., González-Acosta, A. F., De la Cruz-Agüero, J., & Moncayo-Estrada, R. (2006). Ictiofauna marina-costera del Pacífico central mexicano: análisis preliminar de su riqueza y relaciones biogeográficas. In M. C. Jiménez-Quiroz, & E. Espino-Barr (Eds.), Los recursos pesqueros y acuícolas de Jalisco, Colima y Michoacán. Colima: Instituto Nacional de la Pesca, SAGARPA.
Cornish, A. (2004). Stereolepis gigas. The IUCN Red List of Threatened Species 2004: e. T20795A9230697. Retrieved on March 7th, 2017, from: http://www.iucnredlist.org/details/20795/0
Cota-Nieto, J. J., Jiménez-Esquivel, V., & Mascareñas-Osorio, I. (2015). La pesca en Bahía Magdalena Almejas: motor económico para B.C.S. DataMares, Interactive Resource. https://dx.doi.org/10.13022/MM3GW2F
De la Cruz-Agüero, J., Galván-Magaña, F., Abitia-Cárdenas, L. A., Rodríguez-Romero, J., & Gutiérrez-Sánchez, F. J. (1994). Lista sistemática de los peces marinos de Bahía Magdalena, Baja California Sur (México). Ciencias Marinas, 20, 17–31. http://dx.doi.org/10.7773/cm.v20i1.956
Diario Oficial de la Federación (DOF). (2010). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo, Secretaría del Medio Ambiente y Recursos Naturales, México, Retrieved on January 30th, 2023, from: https://www.profepa.gob.mx/innovaportal/file/435/1/NOM_059_SEMARNAT_2010.pdf
Diario Oficial de la Federación (DOF). (2919). Modificación del Anexo Normativo III, Lista de especies en riesgo de la Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo, publicada el 30 de diciembre de 2010. Secretaria del Medio Ambiente y Recursos Naturales, México, 2019. Retrieved on January 30th, 2023, from: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019#gsc.tab=0
Dulvy, N. K., Baum, J. K., Clarke, S., Compagno, L. J. V., Cortés, E., Domingo, A. et al. (2008). You can swim but you can´t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conservation: Marine and Freshwater Ecosystems, 18, 459–482. https://doi.org/10.1002/aqc.975
Etnoyer, P., Canny, D., Mate, B., & Morgan, L. (2004). Persistent pelagic habitats in the Baja California to Bering Sea (B2B) ecoregion. Oceanography, 17, 90–101. https://doi.org/10.5670/oceanog.2004.71
Ferrari, L., López-Martínez, M., Orozco-Esquivel, T., Bryan, S. E., Duque-Trujillo, J., Lonsdale, P. et al. (2013). Late Oligocene to Middle Miocene rifting and synextensional magmatism in the southwestern Sierra Madre Occidental, Mexico: the beginning of the Gulf of California rift. Geosphere, 9,1–40. https://doi.org/10.1130/GES00925.1
Finkbeiner, E. M., & Basurto, X. (2015). Re-defining co-management to facilitate small-scale fisheries reform: an illustration from northwest Mexico. Marine Pollution, 51, 433–441. https://doi.org/10.1016/j.marpol.2014.10.010
Fischer, W., Krupp, F., Schneider, W., Sommer, C., Carpenter, K. E., & Niem, V. H. (1995). Guía FAO para la identificación de especies para los fines de la pesca. Pacífico centro-oriental. Roma, Italy: FAO.
Fricke, R., Eschmeyer, W. N., & Van der Laan, R. (Eds.). (2023). Eschmeyer’s catalog of fishes: genera, species, references. World Wide Web electronic publication. Retrieved on January 30th, 2023, from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Froese, R., & Pauly, D. (Eds.). (2023). FishBase. World Wide Web electronic publication. Retrieved on January 30th, 2023, from: https://www.fishbase.org
Funes-Rodríguez, R., Leal-Espinoza, J. D., Hinojosa-Medina, A., Hernández-Rivas, M. E., & Flores-Coto, C. (2007). Composición, distribución y abundancia de larvas de peces en Bahía Magdalena. In R. Funes-Rodríguez, J. Gómez-Gutiérrez, & R. Palomares-García (Eds.), Estudios ecológicos en bahía Magdalena (pp. 205–219). México D.F.: CICIMAR-IPN.
Galván-Magaña, F., Gutiérrez-Sánchez, F., Abitia-Cárdenas, L. A., & Rodríguez-Romero, J. (2000). The distribution and affinities of the shore fishes of the Baja California Sur lagoons. In M. Manuwar, S. G. Lawrence, I. F. Manuwar, & D. E. Malle (Eds.), Aquatic ecosystems of Mexico: status and scope, ecovision World Monograph Series (pp. 383–398). Leiden, The Netherlands: Backhuys Publishers.
GBIF (Global Biodiversity Information Facility). (2023). Retrieved on January 30th, 2023 (version 02.01/2023), from: http://data.gbif.org
Gómez-González, A. E., Velázquez-Velázquez, E., Rodiles-Hernández, R., González-Díaz, A. A., González-Acosta, A. F., & Castro-Aguirre, J. L. (2012). Lista sistemática de la ictiofauna en la Reserva de la Biosfera La Encrucijada, Chiapas, México. Revista Mexicana de Biodiversidad, 83, 674–686. https://doi.org/10.7550/rmb.24468
Gómez-Valdez, J., & Vélez-Muñoz, H. S. (1982). Variaciones estacionales de la temperatura y salinidad en una región costera de la Corriente de California. Ciencias Marinas, 8, 167–178. https://doi.org/10.7773/cm.v8i2.392
González-Acosta, A. F., Balart, E. F., Ruiz-Campos, G., Espinosa-Pérez, H., Cruz-Escalona, V. H., & Hernández-López, A. (2018). Diversidad y conservación de los peces de la Bahía de La Paz. Baja California Sur, México. Revista Mexicana de Biodiversidad, 89, 705–740. https://doi.org/10.22201/ib.20078706e.2018.3.2145
González-Acosta, A. F., Monsalvo-Flores, E. A., Tovar-Ávila, J., Jiménez-Castañeda, M. F., Alejo-Plata, M. C., & De La Cruz-Agüero, G. (2021). Diversity and conservation of Chondrichthyes in the Gulf of California. Marine Biodiversity, 51, 1–16. https://doi.org/10.1007/s12526-021-01186-9
González-Acosta, A. F., Rabadán-Sotelo, J. A., Ruiz-Campos, G., Del Moral-Flores, L. F., & Borges-Souza, J. M. (2015). A systematic list of fishes from an insular mangrove ecosystem in the Gulf of California. In R. Riosmena-Rodriguez, A. F. González-Acosta, & R. Muñiz-Salazar (Eds.), The arid mangroves from Baja California Peninsula (pp. 81–92). Hauppauge, New York: Nova Science Publishers.
González-Acosta, A. F., Rodiles-Hernández, R., & González-Díaz, A. A. (2018). Checklist of the marine and estuarine fishes of Chiapas, Mexico. Marine Biodiversity, 48, 1439–1454. https://doi.org/10.1007/s12526-016-0630-y
González-Acosta, A. F., Ruiz-Campos, G., & Balart, E. F. (2015). Composition and zoogeography of fishes in mangrove ecosystems of Baja California Sur, México. In R. Riosmena-Rodríguez, A. F. González-Acosta, & R. Muñiz-Salazar (Eds.), The arid mangroves from Baja California Peninsula (pp. 63–80). Hauppauge, New York: Nova Science Publishers.
González-Acosta, A. F., Ruiz-Campos, G., Cruz-Escalona, V. H., & Urcádiz-Cázares, F. J. (2021). Lista comentada de la ictiofauna del estuario del río Mulegé, golfo de California, México. Revista Mexicana de Biodiversidad, 92, e923616. https://doi.org/10.22201/ib.20078706e.2021.92.3616
Hastings, P. A. (2000). Biogeography of the tropical eastern Pacific: distribution and phylogeny of chaenopsid fishes. Zoological Journal of the Linnean Society, 128, 319–335. https://doi.org/10.1111/j.1096-3642.2000.tb00166.x
Hastings, R. H., & Fischer, D. W. (2001). Management priorities for Magdalena Bay, Baja California, México. Journal of Coastal Conservation, 7, 193–202. https://www.jstor.org/stable/25098366
Hastings, P. A., Findley, L. T. & van der Heiden, A. M. (2010). Fishes of the Gulf of California. In R. Brusca (Ed.), The Gulf of California. Biodiversity and conservation (pp. 96–118). Tucson: University of Arizona Press.
Hastings, R. A., Rutterford, L. A., Freer, J. J., Collins, R. A., Simpson, S. D., & Genner, M. J. (2020). Climate change drives poleward increases and equatorial declines in marine species. Current Biology, 30, 1572–1577. https://doi.org/10.1016/j.cub.2020.02.043
Hernández-Velasco, A., Fernández-Rivera Melo, F. J., Melo-Merino, S. M., & Villaseñor-Derbez, J. C. (2016). Occurrence of Holacanthus clarionensis (Pomacanthidae), Stegastes leucorus, and Stegastes acapulcoensis (Pomacentridae) at Magdalena Bay, B.C.S., Mexico. Marine Biodiversity Records, 9, 49. https://doi.org/10.1186/s41200-016-0062-1
Hinojosa-Medina, A., Funes-Rodríguez, R., Aceves-Medina, G., & Gómez-Gutiérrez, J. (2007). Evolución de la investigación científica en el complejo lagunar Bahía Magdalena-Almejas. In R. Funes-Rodríguez, J. Gómez-Gutiérrez, & R. Palomares-García (Eds.), Estudios ecológicos en Bahía Magdalena (pp. 289–311). México D.F.: CICIMAR-IPN.
Hubbs, C. L. (1960). The marine vertebrates of the outer coast. Symposium: The Biogeography of Baja Californian and adjacent seas. Systematic Zoology, 9, 134–147. https://doi.org/10.2307/2411962
IUCN (International Union for Conservation of Nature). (2022). The IUCN Red List of Threatened Species, version 2022-2, 2022. Retrieved on January 30th, 2023, from: http://www.iucnredlist.org
Lea, R. N., & Rosenblatt, R. H. (2000). Observation on fishes associated with the 1997-98 El Niño off California. California Cooperative Oceanic Fisheries Investigations Reports, 41, 117–129.
Lewis, L. R., & Ebeling, P. E. (1971). Baja Sea guide. San Francisco, CA: Miller Freeman Publications.
Love, M. S., & Passarelli, J. K. (2020). Miller and Lea’s guide to the coastal marine fishes of California. Davis, CA: University of California Agriculture and Natural Resources.
Love, M. S., Bizarro, J. J., Cornthwaite, A. M., Frable, B. W., & Maslenikov, K. P. (2021). Checklist of marine and estuarine fishes from the Alaska-Yukon Border, Beaufort Sea, to Cabo San Lucas, Mexico. Zootaxa, 5053, 1–285. https://doi.org/10.11646/ZOOTAXA.5053.1.1
Lluch-Belda, D., Hernández-Rivas, M. E., & Guerrero-Caballero, R. (2000). Variabilidad de la temperatura superficial del mar en Bahía Magdalena, BCS. Oceánides, 15, 1–23.
Malakoff, D. (2004). New tools reveal treasures at ocean hot spots. Science, 304, 1104–1105. https://doi.org/10.1126/science.304.5674.1104
Ojeda-Ruiz, M. A., Marín-Monroy, E. A., Hinojosa-Arango, G., Flores-Irigoyen, A., Cota-Nieto, J. J., Cavieses-Núñez, R. A. et al. (2018). Development of fisheries in Bahía Magdalena-Almejas: the need to explore new policies and management paradigms. Ocean and Coastal Management, 161, 1–10. https://doi.org/10.1016/j.ocecoaman.2018.04.014
Page, L. M., Espinosa-Pérez, H., Findley, L. T., Gilbert, C. R., Lea, R. N., Mandrak, N. E. et al. (2013). Common and scientific names of fishes from the United States, Canada, and Mexico. Bethesda, MD: American Fisheries Society.
Parrish, R., Nelson, H., & Bakun, A. (1981). A. Transport mechanisms and reproductive success of fishes in the California Current. Biological Oceanography, 1, 175–203. https://doi.org/10.1080/01965581.1981.10749438
Robertson, D. R., & Allen, G. R. (2015). Peces costeros del Pacífico Oriental tropical: sistema de información en línea. Ver. 2. Balboa, República de Panamá; Instituto Smithsonian de Investigaciones Tropicales. Retrieved on December 6th, 2022, from: https://www.biogeodb.stri.si.edu/sftep/es/pages.
Rodríguez-Romero, J., López-González, L., Galván-Magaña, F., Gutiérrez-Sánchez, F. J., López-Martínez, J., Inohuye-Riveral, R. et al. (2012). El papel ecológico de los peces en una zona de manglar de la costa occidental de Baja California Sur, México. In J. López-Martínez, & E. Morales-Bojórquez (Eds.), Efectos de la pesca de arrastre en el Golfo de California, México (pp. 93–113). Hermosillo: Centro de Investigaciones Biológicas del Noroeste, S.C./ Fundación Produce Sonora.
Rosenblatt, R. H., & Parr, T. D. (1969). The pacific species of the clinid fish genus Paraclinus. Copeia, 1, 1–20. https://doi.org/10.2307/1441691
Ruiz-Campos, G., Ramírez-Valdez, A., González-Acosta, A. F., Castro-Aguirre, J. L., González-Guzmán, S., & de la Cruz-Agüero, J. (2010). Composition, density and biogeographic affinities of the rocky intertidal fishes on the western coast of the Baja California Peninsula, Mexico. California Cooperative Oceanic Fisheries Investigations Reports, 51,210–220.
Tapia-García, M., Suárez-Núñez, C., Cerdenares-Ladrón de Guevara, G., Macuitl, M. C., & García-Abad, M. C. (1998). Composición y distribución de la ictiofauna en la laguna del Mar Muerto, Pacífico Mexicano. Revista de Biología Tropical, 46, 277–284.
Whitmore, R. C., Brusca, R. C., León-de la Luz, J. L., González-Zamorano, P., Mendoza-Salgado, R., Amador-Silva, E. S. et al. (2005). The ecological importance of mangroves in
Baja California Sur: conservation implications for an endangered ecosystem. In J. L. E. Cartron, G. Ceballos, & R. S. Felger (Eds.), Biodiversity, ecosystems, and conservation in northern Mexico (pp. 298–333). Oxford, London; Oxford University Press. https://doi.org/10.22201/ib.20078706e.2018.3.2145