Priority areas for conservation based on endemic vascular plant species and their biocultural attributes: a case study in Sinaloa, Mexico
C. Rocío Álamo-Herrera a, María Clara Arteaga a, *, Rafael Bello-Bedoy b
a Instituto Politécnico Nacional, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Unidad Durango, Sigma No. 119, Fracc. 20 de Noviembre II, 34234 Victoria de Durango, Durango, Mexico
b Universidad de Guadalajara, Centro Universitario de Ciencias Biológicas y Agropecuarias, Cátedras Conahcyt-Universidad de Guadalajara, Camino Ramón Padilla Sánchez No. 2100, 45200 Zapopan, Jalisco, Mexico
*Corresponding author: d1j17kk@hotmail.com (J.F. Pío-León)
Received: 20 February 2024; accepted: 02 July 2024
Abstract
Endemic vascular plants are one of the main biodiversity indicators used to propose priority conservation areas. The richness of endemic species and corrected and weighted endemism are the most frequently used criteria, while anthropogenic or biocultural factors such as ethnobotanical value or ecological vulnerability are seldom considered. This work proposes priority conservation areas for Sinaloa, Mexico, considering the richness of its endemic species, corrected and weighted endemism, as well as ethnobotanical value, protection status, and the Priority Conservation Index (PCI). The analysis was performed in a 19 × 19 km grid and included 247 records of 78 species. The areas proposed when considering only the richness of endemic species and the weighted endemism coincided with previously known areas of high biodiversity in the state, which are areas of high collection effort and low anthropogenic impact. When considering the ethnobotanical value and protection status, the areas identified included those with greater anthropogenic impact, which contained species of biocultural and economic importance. When the PCI was used, both of these types of regions were identified. We therefore recommend this index as a better indicator to select priority areas.
Keywords: Conservation index; Ebenopsis caesalpinioides; Ethnobotanical value; Protected Natural Areas; Priority species; Stenocereus martinezii
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Áreas prioritarias para la conservación con base en especies de plantas vasculares endémicas y sus atributos bioculturales: un estudio de caso en Sinaloa, México
Resumen
Las plantas vasculares endémicas son uno de los principales indicadores empleados para proponer áreas prioritarias de conservación. La riqueza de especies endémicas y el endemismo ponderado y corregido son frecuentemente incluidos en los análisis, mientras que aspectos antropogénicos o bioculturales como el valor etnobotánico o la vulnerabilidad ecológica son poco considerados. Este trabajo propone áreas prioritarias de conservación para Sinaloa, México, considerando su riqueza de especies endémicas, endemismo ponderado y corregido, así como el valor etnobotánico, estatus de protección e índice prioritario de conservación (IPC). El análisis se realizó en cuadrículas de 19 × 19 km e incluyó 274 registros de 78 especies. Las áreas resultantes, considerando únicamente la riqueza de especies y el endemismo ponderado, coinciden con áreas previamente conocidas por su alta biodiversidad en el estado, mismas que poseen altos esfuerzos de colectas y bajos impactos antropogénicos. Por el contrario, cuando se consideró el valor etnobotánico y el estatus de protección, las áreas prioritarias incluyen zonas con alto impacto antropogénico, pero con presencia de especies con importancia biocultural y valor económico. Empleando el IPC se identificaron ambos tipos de regiones; en consecuencia, recomendamos este índice como un mejor indicador para seleccionar áreas prioritarias.
Palabras clave: Índice de conservación; Ebenopsis caesalpinioides; Valor etnobotánico; Áreas naturales protegidas; Especies prioritarias; Stenocereus martinezii
Introduction
Plants are essential organisms for maintaining the equilibrium of ecosystems and life on Earth. They provide the vast majority of the ecosystem and subsistence services that humans need to survive, including food, medicine, shelter, oxygen, carbon capture, and soil retention. Caring for plants is therefore an act of self-preservation (Raven, 2018). However, over 50% of the terrestrial vegetation on Earth is severely or moderately altered (Bradshaw et al., 2021).
Mexico is the country with the third to fifth highest plant richness, with more than 23,000 species, half of which are endemic (Conabio, 2023a; Villaseñor & Meave, 2022). However, despite 12% of Mexican territory being decreed as Protected Natural Area, it is estimated that between 37 and 50% of the nation’s land area has been impacted by human activities and that the majority of well-conserved areas are located in desert, semi-desert, and high mountain areas that are difficult to access (González-Abraham et al., 2015; Mora, 2019). Two of the largest and most biodiverse ecosystems in the country —dry forest and temperate forest— have suffered total degradation of 37 and 26% of their cover, respectively (Conabio, 2023b; Ulloa-Ulloa et al., 2017). The main causes of this deforestation have been agriculture and infrastructure development, both in Mexico specifically and worldwide (González-Abraham et al., 2015; Laso-Bayas et al., 2022).
One of the main analytical approaches used to propose priority conservation areas is grid analysis, which identifies centers of high biodiversity (“hotspots”) using criteria such as species richness, richness of endemic species, weighted endemism (WE), presence of threatened species, diversity of specific taxa (families or genera), or phylogenetic richness (Gutiérrez-Rodríguez et al., 2022; Maassoumi & Ashouri, 2022; Mehta et al., 2023; Murillo-Pérez et al., 2022; Qin et al., 2022; Sosa & De-Nova, 2012; Vargas-Amado et al., 2020; Villaseñor et al., 2022). The richness of endemic species in particular has the advantage of using a more precise (though smaller) database than the other aforementioned criteria for grid analysis to indicate conservation priority areas.
On the other hand, other indices can be used to propose conservation priority species based on their ethnobotanical or biocultural value, or the degree of threat they face due to use (e.g., Value of Use, Frequency of Use, Conservation Index) (De Lucena et al., 2013; Dhar et al., 2000; Mehta et al., 2023; Pío-León et al., 2023). However, these indices are not usually included in grid richness analyses to select priority conservation areas. These indices weight each species’ value based on its conservation priority, such that a priority conservation area would be determined not just by the total number of species or endemism, but also by their qualities.
Pío-León et al. (2023) compiled a list of the vascular plant species of Sinaloa and proposed some priority conservation areas based on the presence of 2 or more endemic species. In addition, the authors proposed a Priority Conservation Index (PCI) for each species based on its ethnobotanical value and ecological vulnerability, considering characteristics such as its distribution, habitat, and anthropogenic threats. In this index, species with high ethnobotanical value, slow growth (arboreal habit), threatened habitat (near to agricultural zones), and small distribution area (1 or a few known localities), have higher priority than those with no known ethnobotanical value, rapid growth (herbs), inaccessible habitat (cliffs or steep slopes), and wide distribution. The PCI was calculated with the formula:
PCI= D + H + Fv + Am + VE + Vc
where D is distribution; H, habitat; Fv, life form or habit (Spanish abbreviation); Am, degree of threat to their populations; VE, ethnobotanical value, and Vc, commercial value. However, that work did not perform a grid richness analysis to incorporate the values of these indices with traditional algorithms such as WE.
In the present work, we propose priority conservation areas in Sinaloa considering 3 types of algorithms: 1) richness of endemic species, WE, and corrected weighted endemism (CWE); 2) ethnobotanical value, protection status (NOM-059-SEMARNAT-2010 or IUCN) and PCI, and 3) the combination of 1) and 2). We hypothesized that incorporating those anthropogenic and biocultural attributes would modify the priority conservation areas selected since they will not necessarily correspond to the areas of the highest species richness.
Materials and methods
Sinaloa is located in northwestern Mexico, bordered on the east by the Sierra Madre Occidental (SMO) and on the west by the Pacific Ocean. According to Wiken et al. (2011), the main level III ecoregions that compose it are: 1) Sinaloa and Sonora Hills and Canyons with Xeric Shrub and Low Tropical Deciduous Forest (SS-TDF) (50%, 27,568 km2), which is located in the low parts of the SMO; 2) Sinaloa Coastal Plain with Low Tropical Thorn Forest and Wetland (S-TF) (29%, 15,612 km2), located in the lowlands near the coast, from the south-central portion northward; and 3) SMO with Conifer, Oak, and Mixed Forests (PQF) (15.78%, 8,681 km2) in the high parts of the western slope of the SMO (Fig. 1). It has been estimated that 4,000 species of vascular plants, nearly 80 of them endemic, occur in Sinaloa (Pío-León et al., 2023; Vega-Aviña et al., 2021). However, a large part of the coastal territory has been converted to agricultural land (~ 28,000 km2) (INEGI, 2023), resulting in severely fragmented habitats.
The database was based on the file generated by Pío-León et al. (2023) with some updates (Table 1). We incorporated recently described species (until October 2023) and removed species and collections that lacked reliable geographic coordinates. In addition, we prepared a matrix of weighted values considering the ethnobotanical value (E; 1 = documented use, 0 = no documented use), inclusion in a risk category (R) by the NOM-059-SEMARNAT-2010 (Semarnat, 2019) or IUCN (2023) (1 = included in at least 1 category, 0 = not included), and the value of the Conservation Priority Index (PCI), using the values reported by Pío-León et al. (2023) (Table 1). For the PCI, we assigned values according to their quartile position: 4 (upper quartile), 3 (second quartile), 2 (third quartile), and 1 (lower quartile). From these data, we formed 3 analysis groups: 1) biocultural value (E+R; 0 to 2), 2) PCI value (1 to 4), and 3) PCI + R (1 to 5).
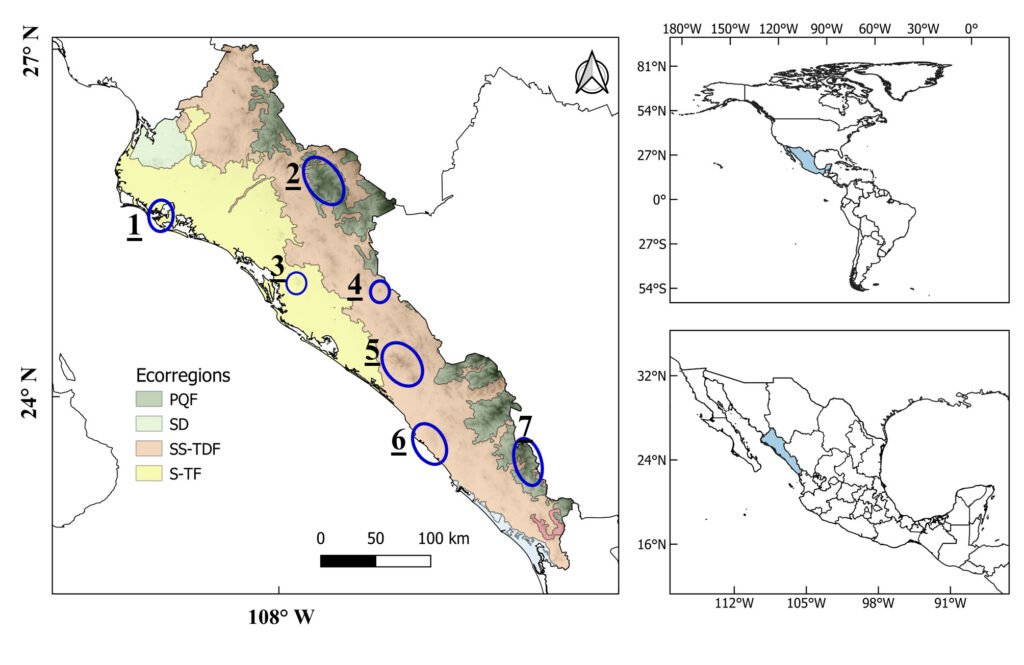
Figure 1. Sinaloa state, Mexico, its main ecoregions level III (Wiken et al., 2011), and the regions of endemism according to Pío-León et al. (2023). Ecoregions: PQF = Conifer, Oak, and Mixed Forests of the Sierra Madre Occidental; SD = Sonoran Desert; SS-TDF = Sinaloa and Sonora Hills and Canyons with Xeric Shrub and Low Tropical Deciduous Forest; S-TF = Sinaloa Coastal Plain with Low Tropical Thorn Forest and Wetlands. Regions of endemism: 1 = Maviri-Topolobampo, 2 = Surutato region, 3 = Cerro Tecomate, 4 = Cerro Colorado, 5 = Sierra Tacuichamona, 6 = Meseta de Cacaxtla, 7 = Sierra de Concordia.
Table 1
List of endemic species of Sinaloa considered for this study and their scores by attributes. E = Ethnobotanical value; R = species with conservation status by the NOM-059-SEMARNAT-2010 or the IUCN (risk); PCI = Priority Conservation Index according to their quartile position.
| Especies | E | R | PCI | E+R | PCI+R |
| Acourtia gentryi L. Cabrera | 0 | 0 | 2 | 0 | 2 |
| Acourtia sinaloana B.L. Turner | 0 | 0 | 1 | 0 | 1 |
| Ageratina concordiana B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Albizia ortegae Britton & Rose | 0 | 0 | 3 | 0 | 3 |
| Aloysia nahuire A.H. Gentry & Moldenke | 1 | 0 | 4 | 1 | 5 |
| Anemia brandegeei Davenp. | 0 | 0 | 1 | 0 | 1 |
| Arachnothryx sinaloae Borhidi | 0 | 0 | 2 | 0 | 2 |
| Bastardiastrum tarasoides Fryxell | 0 | 0 | 2 | 0 | 2 |
| Bastardiastrum wissaduloides (Baker f.) Bates | 0 | 0 | 1 | 0 | 1 |
| Bletia santosii H. Ávila, J.G. González & Art. Castro | 0 | 0 | 2 | 0 | 2 |
| Bourreria franciscoi Pío-León & Vega | 0 | 0 | 3 | 0 | 3 |
| Bourreria ritovegana Pio-León, M.G. Chávez & L.O. Alvarado | 0 | 0 | 3 | 0 | 3 |
| Bouvardia sinaloae Borhidi & E. Martínez | 0 | 0 | 2 | 0 | 2 |
| Calliandra estebanensis H.M. Hern. | 0 | 0 | 2 | 0 | 2 |
| Carlowrightia fuertensis T.F. Daniel | 0 | 0 | 3 | 0 | 3 |
| Castilleja racemosa (Breedlove & Heckard) T.I. Chuang & Heckard | 0 | 0 | 3 | 0 | 3 |
| Chrysactinia lehtoae D.J. Keil | 0 | 0 | 2 | 0 | 2 |
| Cnidoscolus sinaloensis Breckon ex Fern.Casas | 0 | 1 | 3 | 1 | 4 |
| Cochemiea thomasii García-Mor., Rodr. González, J. García-Jim. & Iamonico | 0 | 0 | 2 | 0 | 2 |
| Coutaportla helgae Pío-León, Torr.-Montúfar & H. Ávila | 0 | 0 | 1 | 0 | 1 |
| Coutaportla lorenceana Torr.-Montúfar, H. Ochot. & Art.Castro | 0 | 0 | 2 | 0 | 2 |
| Croton ortegae Standl. | 0 | 0 | 3 | 0 | 3 |
| Ctenodon rosei Morton | 0 | 0 | 3 | 0 | 3 |
| Cuphea delicatula Brandegee | 0 | 0 | 2 | 0 | 2 |
| Cyclanthera monticola Gentry | 0 | 0 | 2 | 0 | 2 |
| Dioscorea sinaloensis O. Téllez | 0 | 0 | 2 | 0 | 2 |
| Dryopetalon breedlovei (Rollins) Al-Shehbaz | 0 | 0 | 1 | 0 | 1 |
| Ebenopsis caesalpinioides (Standl.) Britton & Rose | 1 | 1 | 4 | 2 | 5 |
| Echeveria coppii Moran ex Gideon F.Sm. & Bischofberger | 0 | 0 | 2 | 0 | 2 |
| Echeveria juliana Reyes, González-Zorzano & Kristen | 0 | 0 | 1 | 0 | 1 |
| Echeveria kimnachii J. Meyrán & R. Vega | 0 | 0 | 1 | 0 | 1 |
| Epidendrum petacaense Hágsater, J. Duarte & Pío-León | 0 | 0 | 2 | 0 | 2 |
| Eryngiophyllum rosei Greenm. | 0 | 0 | 2 | 0 | 2 |
| Frangula surotatensis (Gentry) A. Pool | 0 | 0 | 2 | 0 | 2 |
| Graptopetalum sinaloensis Vega | 0 | 0 | 1 | 0 | 1 |
| Guardiola stenodonta S.F. Blake | 0 | 0 | 1 | 0 | 1 |
| Helicteres vegae Cristóbal | 0 | 0 | 3 | 0 | 3 |
| Heliopsis sinaloensis B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Table 1. Continued | |||||
| Especies | E | R | PCI | E+R | PCI+R |
| Hofmeisteria sinaloensis Gentry | 0 | 0 | 1 | 0 | 1 |
| Indigofera sinaloensis M. Sousa & Cruz Durán | 0 | 0 | 2 | 0 | 2 |
| Ipomopsis monticola J.M. Porter & L.A. Johnson | 0 | 0 | 2 | 0 | 2 |
| Iresine arenaria Standl. | 0 | 0 | 1 | 0 | 1 |
| Koanophyllum concordianum B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Lasianthaea gentryi B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Lasianthaea ritovegana B.L. Turner | 0 | 0 | 1 | 0 | 1 |
| Licania mexicana Lundell | 0 | 0 | 2 | 0 | 2 |
| Lobelia macrocentron (Benth.) T.J. Ayers | 0 | 0 | 2 | 0 | 2 |
| Lopezia conjugens Brandegee | 0 | 0 | 1 | 0 | 1 |
| Lopezia sinaloensis Munz | 0 | 0 | 1 | 0 | 1 |
| Lupinus gentryanus C.P. Sm. | 0 | 0 | 2 | 0 | 2 |
| Lupinus howard-scottii C.P. Sm. | 0 | 0 | 2 | 0 | 2 |
| Lupinus sinaloensis C.P. Sm. | 0 | 0 | 2 | 0 | 2 |
| Mariosousa gentryi Seigler & Ebinger | 0 | 0 | 3 | 0 | 3 |
| Mimosa coelocarpa B.L. Rob. | 0 | 0 | 3 | 0 | 3 |
| Mitracarpus aristatus Borhidi & Lozada-Pérez | 0 | 0 | 2 | 0 | 2 |
| Molinadendron sinaloense (Standl. & Gentry) P.K. Endress | 0 | 1 | 3 | 1 | 4 |
| Pavonia gentryi Fryxell | 0 | 0 | 2 | 0 | 2 |
| Peniocereus papillosus (Britton & Rose) U. Guzmán | 0 | 0 | 2 | 0 | 2 |
| Periptera trichostemon Bullock | 0 | 0 | 2 | 0 | 2 |
| Perityle canescens Everly | 0 | 0 | 1 | 0 | 1 |
| Perityle grandifolia Brandegee | 0 | 0 | 1 | 0 | 1 |
| Perityle stevensii B.L. Turner | 0 | 0 | 1 | 0 | 1 |
| Physalis vestita Waterf. | 0 | 0 | 3 | 0 | 3 |
| Pitcairnia monticola Brandegee | 0 | 0 | 1 | 0 | 1 |
| Polygala polyedra Brandegee | 0 | 0 | 1 | 0 | 1 |
| Psacalium quercifolium H.Rob. & Brettell | 0 | 0 | 2 | 0 | 2 |
| Salvia beltraniorum J.G.González, Pío-León & Art.Castro | 0 | 0 | 2 | 0 | 2 |
| Salvia trichostephana Epling | 0 | 0 | 2 | 0 | 2 |
| Sedum copalense Kimnach | 0 | 0 | 1 | 0 | 1 |
| Stenocereus martinezii (J.G. Ortega) Bravo | 1 | 1 | 4 | 2 | 5 |
| Stevia concordiana B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Sysyrinchium jacquelineanum Art.Castro, H. Ávila & J.G. González | 0 | 0 | 1 | 0 | 1 |
| Tibouchina thulia Todzia | 0 | 0 | 1 | 0 | 1 |
| Tillandsia mazatlanensis Rauh | 0 | 0 | 2 | 0 | 2 |
| Tillandsia occulta H. Luther | 0 | 0 | 2 | 0 | 2 |
| Verbesina microcarpa S.F. Blake | 0 | 0 | 2 | 0 | 2 |
| Verbesina ortegae S.F. Blake | 0 | 0 | 2 | 0 | 2 |
| Verbesina sinaloensis B.L. Turner | 0 | 0 | 2 | 0 | 2 |
Richness of endemism (SR), weighted endemism (WE), and corrected weighted endemism (CWE). The richness of endemic species was quantified in 19 × 19 km cells (361 km2), dividing Sinaloa into 195 cells. The cell size used was determined according to the criterion of Oyala (2020). Endemic species richness was quantified as the total number of endemic species whose distribution includes the cell. Endemism was evaluated using the WE and CWE indices. The WE score for each cell was obtained by summing, for each species present in the cell, the inverse of the number of cells in which the species occurs; thus, a high WE value indicates cells that contain more species with restricted distributions (i.e., that are found in few other cells), while low WE values indicate cells that mostly contain widely distributed species (i.e., species that are also present in other cells). The CWE is similar, but additionally corrects for potential biases due to differences in overall richness by dividing the value of the WE by the number of species present in the cell (Laffan & Crisp, 2003). The 3 parameters (SR, WE, and CWE) were estimated in the program Biodiverse v.2.0 (Laffan et al., 2010). Geoprocessing of the data was performed in QGIS 3.4.8 (QGIS.org, 2019).
Endemism weighted by biocultural attributes and PCI. In addition to SR, WE, and CWE analysis, endemism weighted by biocultural attributes was evaluated using 2 sets of attribute/parameter combinations, each resulting in 3 maps, 9 in total (Fig. 2). The first set included the species richness plus the biocultural values, resulting in the following 3 combinations: species richness plus biocultural value (SR+E+R), species richness plus PCI (SR+PCI), and PCI plus the risk category (SR+PCI+R). The second set did not consider species richness, resulting in the combinations of biocultural value (E+R), PCI, and PCI+R. For this second set of analyses, only species that fulfilled the relevant criteria were included (e.g., the E+R combination included only species that had ethnobotanical value and are included in a risk category). As such, in the first set of maps, a priority conservation area depended by the number of species present and their qualities (e.g., species with ethnobotanical value or species with protected status), while in the second only the species’ qualities were considered.
The final priority conservation areas were based on the consensus map of the 9 different endemism maps. The consensus areas took into account only the cells that had the highest possible value of the relevant variables in at least 1 of the 9 previously generated endemism maps. The consensus values were obtained by summing the number of times each cell had the highest possible value in each of the endemism maps, such that the highest possible consensus value was theoretically 9 (the cell had the highest possible value in all maps), and the minimum value was 1 (maximal value in only 1 map). The consensus map was also overlayed with Protected Natural Areas and Priority Terrestrial Regions, land use, and bioclimatic corridors.
Results
Occurrence, conservation (risk) status, and ethnobotanical uses of the endemic species of Sinaloa. The database contained 247 records of 78 species, 30 families, and 61 genera. For 48 of the genera (78.7%), only 1 species of the genus was present. The majority of the records were distributed in the central to the southern region of the state, near the coast, in the Meseta de Cacaxtla Natural Protected Area and the area between the former and Sierra de Tacuichamona, as well as in the Concordia and Surutato mountains of the SMO (Fig. 3; regions 6, 5, and 2, in Figure 1). The 2 level III ecoregions best represented were SS-TDF (166 records/ 40 species) and the PQF (53/ 37), followed by the S-TF (17/ 8) (Fig. 3a). Sixty-nine percent of the records fell outside of the polygons of Protected Natural Areas or Priority Terrestrial Conservation Regions (Fig. 3B). Sixty-eight percent of the species (53) were known from a single locality (either a single collection or collections from locations that are very close to each other).
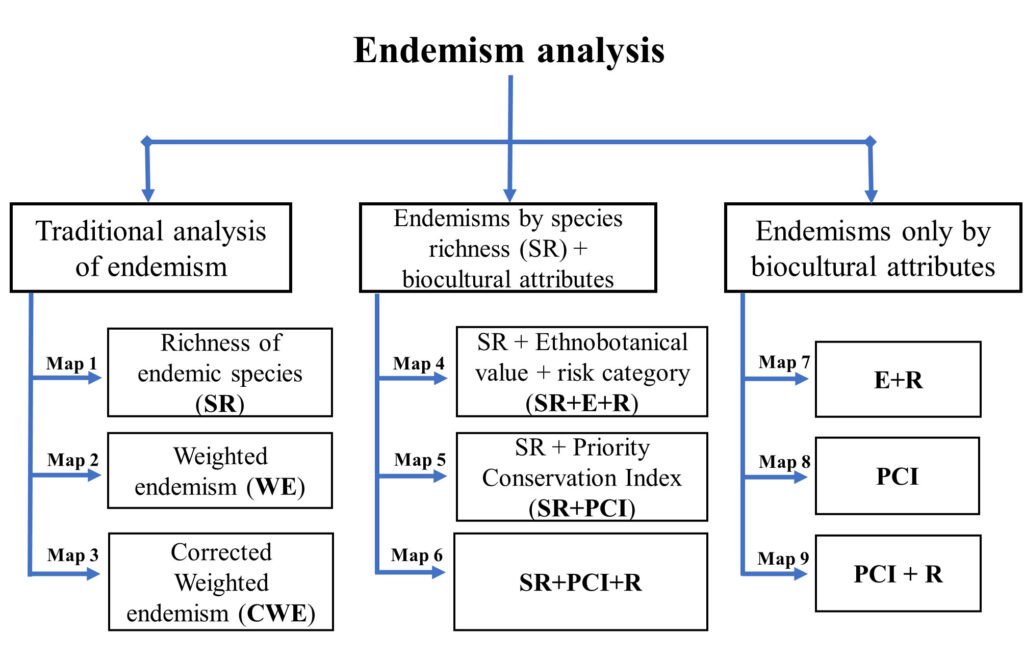
Figure 2. Flowchart of the endemism analysis to select priority areas in Sinaloa, Mexico.
Only 4 species (Cnidoscolus sinaloensis, Ebenopsis caesalpinioides, Molinadendron sinaloense, and Stenocereus martinezii) of the 78 analyzed are found in some risk category (Table 1). All 4 are considered endangered (EN) by the IUCN, while only Stenocereus martinezii is included in NOM-059-SEMARNAT-2010, under the category of special protection (Pr). Only 3 species have well-documented ethnobotanical uses: Aloysia nahuire (aromatic and medicinal tea), Ebenopsis caesalpinioides (edible seeds, occasional commercial value), and Stenocereus martinezii (edible fruits, commercial value). One additional species, Lupinus gentryianus, was noted in the type collection to be used as an anti-parasitic for livestock; however, this plant is only known from that locality, and this use has not since been confirmed, so it was not considered.
The different patterns of endemism are shown in Figure 4. The overall richness of endemism (Fig. 4A) showed 2 main areas —1 in the northern part of the Sierra de Concordia (region 7, Fig. 1) and the other in the western part of the Sierra de Tacuichamona (region 5, Fig. 1)— as well as 3 secondary areas located in the Sierra de Surutato (region 2, Fig. 1), Cerro Colorado (region 4, Fig. 1), and the southern part of the Sierra de Concordia. The WE (Fig. 4B) showed a similar pattern in richness but with an increase in the priority levels of the Sierra de Surutato and a decrease by 1 level for the Tacuichamona and Cerro Colorado. The CWE (Fig. 4C) showed several priority areas more scattered across the state than the WE, mainly in the SMO, corresponding to the majority of the species known from a single locality; however, compared with WE, there was a greater concentration of high-priority cells toward the northern part of the state, near southern Sonora, in the area around the Sierra de Barobampo and Hills of Topolobampo (region 1, Fig. 1).

Figure 3. Records of endemic species in Sinaloa overlayed onto: level III ecoregions (Wiken et al., 2011; definitions in Figure 1) (A) and Protected Natural Areas (PNA) and Priority Terrestrial Regions (B). Categories of Protected Natural Areas: PNAS = state; PNAM = municipal; PNAF = federal; PTR = Priority Terrestrial Regions.
Figure 4. Endemism areas of vascular plants in Sinaloa, Mexico, according to the calculated index values (A-I): SR = endemic species richness; WE = weighted endemism; CWE = corrected weighted endemism; E = ethnobotanical value; R = species with protection status; PCI = Priority Conservation Index.
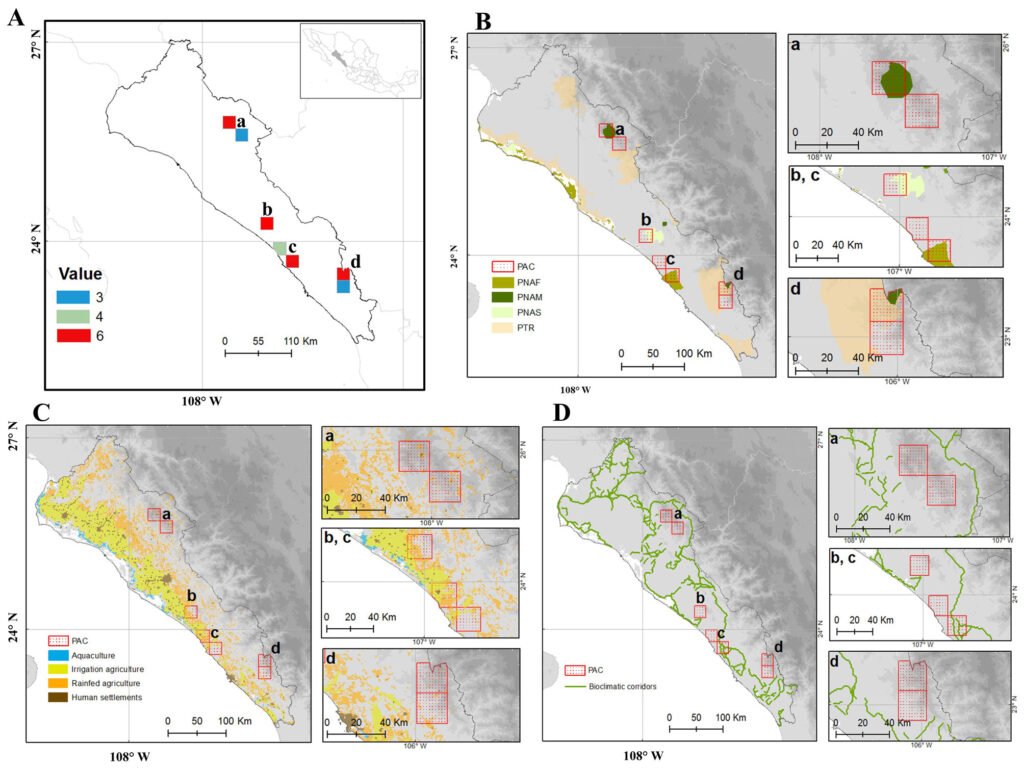
Figure 5. Consensus priority conservation areas (PAC) in the state of Sinaloa (A-D). Consensus map (A) superimposed to: Protected Natural Areas/Priority Terrestrial Regions (B), land use (C), and bioclimatic corridors (D). Categories of Protected Natural Areas: PNAS = state; PNAM = municipal; PNAF = federal; PTR = Priority Terrestrial Regions.
The addition of the ethnobotanical attributes to the protection status and richness of endemic species (SR+E+R) (Fig. 4D) showed an increase in the values for the areas from the Meseta de Cacaxtla (region 6, Fig. 1) to Sierra de Tacuichamona, but a decrease in the zones of the SMO. Adding the Priority Conservation Index to the richness (SR+PCI) (Fig. 4E) showed an increase and homogenization of the priority in all of the aforementioned regions, while adding protection status (SR+PCI+R) (Fig. 3F) did not significantly modify the areas of importance.
Finally, when considering only the ethnobotanical value plus the protection status (E+R), without considering species richness (i.e., eliminating the species that did not have those attributes), the zone of highest priority was concentrated nearly exclusively in the southern part of the state, within and adjacent to the Meseta de Cacaxtla (Fig. 3G). When considering PCI only or PCI plus risk category, there was again a homogenization of the high priority for the 2 mountainous areas (Surutato and Concordia), Meseta de Cacaxtla, Tacuichamona, and surrounding areas (Fig. 4H, I).
The priority conservation areas, as defined by the consensus among the 9 maps analyzed, were composed of 7 polygons grouped into 3 categories (Fig. 5): 4 cells with a value of 6 (of the maximum possible score of 9) in the northern part of the Sierra de Concordia, northwestern part of Sierra Surutato, Meseta de Cacaxtla, and Sierra de Tacuichamona; 1 with a value of 4 in the area between the Meseta de Cacaxtla and Tacuichamona; and 2 with a value of 3 in the southern part of the Sierra de Concordia and southeastern part of the Sierra de Surutato (Fig. 5A). However, since the 3 areas with a value of 3 or 4 were contiguous with areas with a value of 6, 4 priority conservation areas were proposed: Sierra de Surutato (Fig. 45-a), Sierra de Tacuichamona (Fig. 5A-b), Meseta de Cacaxtla (Fig. 5A-c), and Sierra de Concordia (Fig. 5A-d).
Superimposing the consensus map with the map of existing Protected Natural Areas (Fig. 5B) showed that these 4 consensus areas fall partially within protected areas: 1 federal (Área de Protección de Flora y Fauna Meseta de Cacaxtla, Fig. 5B-c), 1 state (Sierra de Tacuichamona, Fig. 5B-b), and 2 municipal (Reserva Chara Pinta in the Sierra de Concordia and Reserva de Surutato, Fig. 5B-d, B-a, respectively). The Sierra de Concordia also includes part of the terrestrial priority region Río Presidio. Regarding land use, the 2 consensus areas in the SMO were found in mixed pine-oak forest with low impact of agricultural activity (Fig. 5C-a, C-d), while the other 2, located in the Sinaloa and Sonora Hills and Canyons with Xeric Shrub and Low Tropical Deciduous Forest ecoregion, present moderate to high impact from irrigated and rainfed agriculture (Fig. 5C-b, C-c). When considering biological corridors, only the priority area in the Meseta de Cacaxtla overlapped with a bioclimatic corridor.
Discussion
The analyses of richness of endemic species and WE showed higher conservation priority in areas that were previously identified as having high endemism (Pío-León et al., 2023), low anthropogenic impact from agriculture, and which have also historically been subject to concentrated collection efforts (Sierra de Surutato and Sierra de Concordia) (Ávila-González et al., 2019; Gentry 1946; Vega-Aviña et al., 2021). On the other hand, the regions defined based on CWE reflected a high number of species known from a single locality, which could indicate the presence of small islands of endemism in the state or low collection effort. In contrast, the inclusion of the ethnobotanical criteria and protection status (E+R) shows a different pattern from species richness, concentrating high priority scored in an area of transition between the coastal plain of Sinaloa and the hills of Sinaloa and Sonora, near the coast in the center-south of the state. These regions correspond to the transition and ecotone between low tropical deciduous forest and thorn forest, which are strongly impacted by anthropogenic activities (irrigated and rainfed agriculture), suggesting that the species with the highest ethnobotanical importance and with protected status (IUCN or NOM-053-SEMARNAT-2010) are found near human activities that require stronger conservation attention than those located in the high parts of the SMO, where the threats are less severe.
The priority conservation areas indicated by the consensus map (Fig. 5) include the regions with the highest richness of endemic species plus the areas with the highest number of species with biocultural importance. These consensus areas are practically the same as those that were assigned the highest priority values when considering only the Priority Conservation Index (PCI) for each species; as such, this index was the most robust single indicator for selecting priority conservation areas. This index combines ethnobotanical parameters such as species’ uses and economic value with ecological parameters such as their distribution, habit, and habitat. Thus, it covers a broad range of criteria that are useful for defining priority species or areas for conservation.
All the priority conservation areas defined by the consensus map (Fig. 5) except 1 included part of a Protected Area polygon, although only 1 was under federal jurisdiction (Meseta de Cacaxtla). The only cell that did not overlap with a Protected Natural Area was adjacent to the Meseta de Cacaxtla, and it was the cell with the largest area of agriculture. This area is important because it contains the 2 species with the highest ethnobotanical value (Ebenopsis caesalpinioides and Stenocereus martinezii), which are also found in a risk category according to the IUCN and NOM-053-SEMARNAT-2010. This area therefore urgently requires conservation and restoration activities, especially for E. caesalpinioides, whose distribution is limited to the area surrounding this cell (Pío-León et al., 2023). Specifically, we recommend avoiding the conversion from rainfed agriculture to technified irrigated agricultural activities, since these are generally more aggressive toward native vegetation. This area is also important because it is located at the transition between lowland deciduous forest and thorn forest of Sinaloa, which could reflect high endemism, in addition to potentially serving as part of the bioclimatic corridor connecting the 2 most important terrestrial ANPs in the state, Meseta de Cacaxtla (federal) and Sierra Tacuichamona (state).
In the present study, the incorporation of the species’ biocultural parameters modified the priority areas for conservation compared to the areas selected when considering only the richness of endemic species, weighted endemism, or corrected weighted endemism. Specifically, the richness analysis identified priority areas in the mountainous and high-diversity regions of Sinaloa, while the ethnobotanical and ecological factors incorporated zones near the coast that have higher anthropogenic impact. The Conservation Priority Index identified all of these priority regions; for this reason, we propose it as a complete and robust index for identifying priority conservation areas. At the state level, we recommend that conservation and restoration actions be implemented in the area of transition between the low tropical deciduous forest and thorn forest. This area simultaneously presents the highest impact of anthropogenic activities and harbors the most important Sinaloa endemic species in terms of biocultural value and protection status —the “pitaya de Sinaloa” (Stenocereus martinezii) and the “guampinola” or “frutilla” (Ebenopsis caesalpinioides). This area should be considered a priority for both conservation and restoration, which would not have been identified as a priority if only the richness of endemism or CWE had been analyzed.
Acknowledgements
The first author is grateful to the Consejo Nacional de Humanidades, Ciencia y Tecnología (Conahcyt) for the grant awarded as part of the Estancias Posdoctorales por México program (I1200/320/2022). We also thank Jorge David López Pérez for his suggestions on data analysis, and the two anonymous reviewers for their comments and suggestions that improved our manuscript.
References
Ávila-González, H., González-Gallegos, J. G., López-Enríquez, I. L., Ruacho-González, L., Rubio-Cardoza, J., & Castro-Castro, A. (2019). Inventario de las plantas vasculares y tipos de vegetación del Santuario El Palmito, Sinaloa, México. Botanical Sciences, 97, 789–820. https://doi.org/10.17129/botsci.2356
Bradshaw, C. J. A., Ehrlich, P. R., Beattie, A., Ceballos, G., Crist, E., Diamond, J. et al. (2021). Underestimating the challenges of avoiding a ghastly future. Frontiers in Conservation Sciences, 1, 615419. https://doi.org/10.3389/fcosc.2020.615419
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2023a). México megadiverso. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Ciudad de México. Retrieved 01 December, 2023 from: https://www.biodiversidad.gob.mx/pais/quees
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2023b). Ecosistemas de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Ciudad de México. Retrieved 01 December, 2023 from: https://www.biodiversidad.gob.mx/ecosistemas/ecosismex
Dhar, U., Rawal, R. S., & Upreti, J. (2000). Setting priorities for conservation of medicinal plants ––a case study in the Indian Himalaya. Biological Conservation, 95, 57–65. https://doi.org/10.1016/S0006-3207(00)00010-0
De Lucena, R. F., Lucena, C. M., Araújo, E. L., Alves, Â. G., & Albuquerque, U. P. D. (2013). Conservation priorities of useful plants from different techniques of collection and analysis of ethnobotanical data. Anais da Academia Brasileira de Ciências, 85,169–186. https://doi.org/10.1590/S0001-37652013005000013
Gentry, H. S. (1946). Notes on the vegetation of Sierra Surotato in northern Sinaloa. Bulletin of the Torrey Botanical Club, 73, 451–462. https://doi.org/10.2307/2481592
González-Abraham, C., Ezcurra, E., Garcillán, P. P., Ortega-Rubio, A., Kolb, M., & Bezaury Creel, J. E. (2015). The human footprint in Mexico: physical geography and historical legacies. Plos One, 10, e0121203. https://doi.org/
10.1371/journal.pone.0121203
Gutiérrez-Rodríguez, B. E., Vásquez-Cruz, M., & Sosa, V. (2022). Phylogenetic endemism of the orchids of Megamexico reveals complementary areas for conservation. Plant Diversity, 44, 351–359. https://doi.org/10.1016/j.pld.20
22.03.004
IUCN (International Union for Conservation of Nature). (2023). The IUCN Red List of Threatened Species. Version 2022-2. Retrieved 01 December, 2023 from: https://www.iucnredlist.org
INEGI (Instituto Nacional de Estadística, Geografía e Informática). (2023). Censo agropecuario 2022. Resultados definitivos Sinaloa. Retrieved 25 January, 2024 from: https://www.inegi.org.mx/
Laso-Bayas, J. C., See, L., Georgieva, I., Schepaschenko, D., Danylo, O., Dürauer, M. et al. (2022). Drivers of tropical forest loss between 2008 and 2019. Scientific Data, 9, 1–8. https://doi.org/10.22022/nodes/06-2021.122
Laffan, S. W., & Crisp, M. D (2003) Assessing endemism at multiple spatial scales, with an example from the Australian vascular flora. Journal of Biogeography, 30, 511–520. https://doi.org/10.1046/j.1365-2699.2003.00875.x
Laffan, S.W., Lubarsky, E., & Rosauer, A. F. (2010) Biodiverse, a tool for the spatial analysis of biological and related diversity. Ecography, 33, 643–647. https://doi.org/10.1111/
j.1600-0587.2010.06237.x
Maassoumi, A. A., & Ashouri, P. (2022). The hotspots and conservation gaps of the mega genus Astragalus (Fabaceae) in the Old-World. Biodiversity and Conservation, 31, 2119–2139. https://doi.org/10.1007/s10531-022-02429-2
Mehta, P., Bisht, K., Sekar, K. C., & Tewari, A. (2023). Mapping biodiversity conservation priorities for threatened plants of Indian Himalayan Region. Biodiversity and Conservation, 32, 2263–2299. https://doi.org/10.1007/s10531-023-02604-z
Mora, F. (2019). The use of ecological integrity indicators within the natural capital index framework: The ecological and economic value of the remnant natural capital of México. Journal for Nature Conservation, 47, 72–92. https://doi.org/10.1016/j.jnc.2018.11.007
Murillo-Pérez, G., Rodríguez, A., Sánchez-Carbajal, D., Ruiz-Sánchez, E., Carrillo-Reyes, P., & Munguía-Lino, G. (2022). Spatial distribution of species richness and endemism of Solanum (Solanaceae) in Mexico. Phytotaxa, 558, 147–177. https://doi.org/10.11646/phytotaxa.558.2.1
Oyala, V. (2020). Sistemas de Información Geográfica. Retrie-
ved on December 25th, 2024 from: http://volaya.es/writing
Pío-León, J. F., González-Elizondo, M., Vega-Aviña, R., González-Elizondo, M. S., González-Gallegos, J. G., Salomón-Montijo, B. et al. (2023). Las plantas vasculares endémicas del estado de Sinaloa, México. Botanical Sciences, 101, 243–269. https://doi.org/10.17129/botsci.3076
QGIS Development Team (2019) Geographic Information System, version 3.4.8. QGIS Association. Retrieved 11 December, 2019 from: http://www.qgis.org
Qin, F., Xue, T., Yang, X., Zhang, W., Wu, J., Huang, Y. et al. (2022). Conservation status of threatened land plants in China and priority sites for better conservation targets: distribution patterns and conservation gap analysis. Biodiversity and Conservation, 31, 2063–2082. https://doi.org/10.1007/s10531-022-02414-9
Raven, P. H. (2018). Saving plants, saving ourselves. Plants People Planet, 1, 8–13. https://doi.org/10.1002/ppp3.3
Semarnat (Secretaría de Medio Ambiente y Recursos Naturales). (2019). Norma Oficial Mexicana NOM-059-SEMARNAT-2010. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación, Ciudad de México. Retrieved 01 December, 2023 from: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019#gsc.tab=0
Sosa, V., & De-Nova, J. (2012). Endemic angiosperm lineages in Mexico: hotspots for conservation. Acta Botanica Mexicana, 100, 293–315. https://doi.org/10.21829/abm100.2012.38
Ulloa-Ulloa, C., Acevedo-Rodríguez, P., Beck, S., Belgrano, M. J., Bernal, R., Berry, P. E. et al. (2017). An integrated assessment of the vascular plant species of the Americas. Science, 358, 1614–1617. https://doi.org/10.1126/science.aao
0398
Vargas-Amado, G., Castro-Castro, A., Harker, M., Vargas-Amado, M. E., Villaseñor, J. L., Ortiz, E. et al. (2020). Western Mexico is a priority area for the conservation of Cosmos (Coreopsideae, Asteraceae), based on richness and track analysis. Biodiversity and Conservation, 29, 545–569. https://doi.org/10.1007/s10531-019-01898-2
Vega-Aviña, R., Vega-López, I. F., & Delgado-Vargas, F. (2021). Flora nativa y naturalizada de Sinaloa. Culiacán, Sinaloa: Universidad Autónoma de Sinaloa.
Villaseñor, J. L., & Meave, J. A. (2012). Floristics in Mexico today: insights into a better understanding of biodiversity in a megadiverse country. Botanical Sciences, 100, S14–S33. https://doi.org/10.17129/botsci.3050
Villaseñor, J. L., Ortiz, E., & Hernández-Flores, M. M. (2022). The vascular plant species endemic or nearly endemic to Puebla, Mexico. Botanical Sciences, 101, 1207–1221. https://doi.org/10.17129/botsci.3299
Wiken, E., Jiménez-Nava, F., & Griffith, G. (2011). North American Terrestrial Ecoregions-Level III. Montreal, Canada: Commission for Environmental Cooperation. Retrieved Jul 17, 2021 from: http://www3.cec.org/islando
ra/en/item/10415-north-american-terrestrial-ecoregionsle
vel-iii