A new species of the genus Monstrilla (Copepoda: Monstrilloida: Monstrillidae) from the Gulf of California, Mexico
Eduardo Suárez-Morales a, *, Karl E. Velázquez-Ornelas b
a El Colegio de la Frontera Sur, Avenida Centenario Km 5.5, 77014 Chetumal, Quintana Roo, Mexico
b Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Mazatlán, Joel Montes Camarena s/n, 82040 Mazatlán, Sinaloa, Mexico
*Corresponding author: esuarez@ecosur.mx (E. Suárez-Morales)
Received: 23 September 2023; accepted: 24 June 2024
http://zoobank.org/urn:lsid:zoobank.org:pub:55476587-382E-4014-9EB2-F111CFF305DC
Abstract
Based on deep-water (700-750 m) biological samples obtained from the southern Gulf of California, Pacific coast of Mexico, a new species of the monstrilloid copepod genus Monstrilla Dana, 1849 is described based on a single subadult female collected close to the bottom with an epibenthic sledge. Monstrilla hendrickxi sp. n. is distinguished by a unique combination of characters including: 1) no trace of eyes; 2) strong, thick antennules with segments 2-4 partly fused; 3) strongly developed apical elements on antennular segment 5 and spinous processes on segments 2-5; and 4) bilobed fifth legs with 2 apical setae on the exopodal lobe and a digitiform, unarmed endopodal lobe. The new species exhibits some affinity with surface-dwelling species of Monstrilla that have 2 setae on the exopodal lobes of the fifth legs. This is the fifth record of a species of the order Monstrilloida in the Gulf of California. The present discovery in deep oceanic waters significantly adds to our knowledge of the habitat range of this copepod order and likely anticipate further interesting findings of monstrilloids in deep waters worldwide.
Keywords: Parasitic copepods; Taxonomy; Sledge; Zooplankton; Monstrillids
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Una especie nueva del género Monstrilla (Copepoda: Monstrilloida: Monstrillidae)
del golfo de California, México
Resumen
Con base en muestras biológicas de aguas profundas (700-750 m) obtenidas del sur del golfo de California, costa del Pacífico de México, se describe una especie nueva del género Monstrilla Dana, 1849 con base en una hembra preadulta recolectada con un trineo epibentónico. Monstrilla hendrickxi sp. n. se distingue por una combinación única de caracteres que incluyen: 1) ausencia de estructuras oculares; 2) anténulas robustas, con los segmentos 2-4 parcialmente fusionados; 3) anténula con elementos apicales muy desarrollados en el segmento 5 y procesos espiniformes en los segmentos 2-5; y 4) quinta pata bilobulada, lóbulo exopodal armado con 2 setas apicales, lóbulo endopodal digitiforme, desarmado. La nueva especie tiene afinidad con congéneres de superficie que tienen lóbulos exopodales armados con 2 setas. Este es el quinto registro de monstriloides en el golfo de California. El presente hallazgo aumenta significativamente nuestro conocimiento sobre el intervalo de hábitats de este orden de copépodos y probablemente anticipa más descubrimientos interesantes de monstriloides en aguas profundas de todo el mundo.
Palabras clave: Copépodos parásitos; Taxonomía; Trineo colector; Zooplancton; Monstrílidos
Introduction
Members of the copepod order Monstrilloida Sars, 1901 are, as larvae, endoparasites of benthic marine invertebrates, but their infective early nauplii and their non-feeding reproductive adult and preadult stages are planktonic. The known hosts of the postnaupliar and juvenile stages include benthic polychaetes (species of the families Syllidae, Capitellidae, Serpulidae, and Spionidae), mollusks, and sponges (Huys et al., 2007; Jeon et al., 2018; Suárez-Morales, 2011, 2018; Suárez-Morales et al., 2010, 2014). In plankton, monstrilloids have been reported chiefly from a wide range of shallow coastal habitats including estuaries (Suárez-Morales et al., 2020), coastal embayments (Suárez-Morales, 1994a, b; Suárez-Morales & Gasca, 1990), and coral reefs (Sale et al., 1996), where they can be found in aggregations (Suárez-Morales, 2001). Monstrilloids have been recently reported from rocky shore tidepools as well (Cruz-Lopes de Rosa et al., 2021). Deep oceanic waters, though, would seem to be an unlikely source of monstrilloid specimens for taxonomic study because of the copepods’ limited dispersal capacity in the water column and their need to remain close to their benthic hosts (Suárez-Morales, 2001, 2011, 2018).
The order is currently represented by a single family Monstrillidae Dana, 1849 containing at least 7 valid genera: Monstrilla Dana, 1849; Cymbasoma Thompson, 1888; Monstrillopsis Sars, 1921; Maemonstrilla Grygier & Ohtsuka, 2008; Australomonstrillopsis Suárez-Morales & McKinnon, 2014; Caromiobenella Jeon, Lee & Soh, 2018, and Spinomonstrilla Suárez-Morales, 2019 (Jeon et al., 2016, 2018; Suárez-Morales et al., 2020). Currently, the genus Cymbasoma, with 78 nominal species, is the most diverse within the order (Razouls et al., 2023: Suárez-Morales & McKinnon, 2016; Walter & Boxshall, 2022). The taxonomic examination of a monstrilloid subadult female collected with a deep-water epibenthic sledge operated close to the bottom within a depth range of 700-750 m in oceanic waters of the southern Gulf of California, Mexico, allowed us to recognize this individual as representative of an undescribed species of Monstrilla, which is herein described following upgraded morphological standards and compared with congeneric species (Grygier & Ohtsuka, 1995). This is the third published record of monstrilloid copepods from deep oceanic waters worldwide (see Suárez-Morales & Mercado-Salas, 2023) and the fifth record of monstrilloids in the Gulf of California.
Materials and methods
A subadult female of a monstrilloid copepod was collected with an epibenthic sledge operated in mid-water, close to the bottom off the Pacific coast of northwestern Mexico, in the Gulf of California. The maximum sampling depth was 750 m. Immediately after collection, the organisms from the haul were preserved in 4% formalin with seawater. Sorting of specimens and preliminary observations were made under an Olympus SZ51 stereomicroscope. The single female monstrilloid individual thus found was recognized as a subadult female and tentatively identified as a member of the genus Monstrilla. Further examination was performed under an Olympus BX51 compound microscope with Nomarski DIC optics. Prior to examination, the specimen was partially dissected and swimming legs 1-4 and the cephalothorax, urosome +antennules were mounted in glycerol on glass slides, which were sealed with acrylic nail varnish. The slides have been deposited in the collection of Zooplankton (ECO-CHZ) held at El Colegio de la Frontera Sur (ECOSUR), Unidad Chetumal, in Chetumal, Quintana Roo, Mexico (ECO-CH-Z). Detailed examination allowed us to determine that this specimen represented an undescribed species, which we describe herein following current descriptive standards in monstrilloid taxonomy. The general morphological terminology follows Huys and Boxshall (1991), while the nomenclature of the antennular armature follows Grygier and Ohtsuka (1995).
Description
Order Monstrilloida Sars, 1901
Family Monstrillidae Dana, 1849
Genus Monstrilla Dana, 1849
Monstrilla hendrickxi sp. nov.
(Figs. 1-4)
http://zoobank.org/urn:lsid:zoobank.org:act:708E6CBF-
9C0B-433B-AF6B-5D474B2490E3
Diagnosis. Large (2.9 mm) female subadult Monstrilla with robust, cylindrical cephalothorax representing about 60% of total body length and no trace of eyes. Antennules thick, about 1/3 as long as cephalothorax; antennulary segments 2-4 partly fused, segment 3 with modified setae, segments 2, 4, and 5 each furnished with a conical or spinous process. Segments 4-5 partly fused, latter segment with strongly developed apical spiniform elements. Ventral side of genital double-somite carrying short, corrugate ovigerous spines not reaching beyond distal end of caudal rami. Fifth leg bilobed, exopodal lobe bearing 2 subequally long terminal setae and endopodal lobe digitiform, unarmed. Caudal rami each with 6 setae, innermost of which (VI) shortest and outer proximal one (I) longest.
Description of holotype subadult female. Body robust; shape and tagmosis as usual in female Monstrilla (Suárez-Morales 1994a, 2019). Total body length 2.92 mm, measured from anterior end of cephalothorax to posterior margin of anal somite. Cephalothorax length 1.74 mm, representing about 60% of total body length, containing thick egg mass (Fig. 1A). Oral cone located 0.53 of way back along ventral surface of cephalothorax. Cephalic region with weakly produced forehead (Fig. 2A). All 3 cups of naupliar eye with pigment absent (Figs. 1A, B, 2A, 3D). Preoral area with ventral ornamentation of small, nipple-like cuticular processes (nlp) with adjacent fields of integumental wrinkles and anterior cluster of pores (apc) (Figs. 3D, 4B). Antennules slender, relatively short (588 µm) and thick, corresponding to 33% of cephalothorax length and almost 20 % of total body length (Fig. 1A). Antennules indistinctly 5-segmented (1-5 in Fig. 1B), segment 1 separate but segments 2-5 partly fused (Figs. 1B, 3A), with intersegmental division 2-3 marked by weak constriction. In terms of current nomenclature for antennular armature of female monstrilloid copepods (Grygier & Ohtsuka, 1995), element 1 present on first segment (Fig. 3A). Second segment armed as usual with elements 2v1-3, 2d1,2, and long element IId (Figs. 3A, B, 4C), but additionally with conical process on distal inner margin (arrowed in Fig. 3B). Putative third segment with setiform element 3, but pair of short, spiniform elements replacing usual long, setiform elements IIIv and IIId (Fig. 3A). Putative fourth segment armed with elements 4v1-3, long 4aes, and elements IVd and IVv (Fig. 3A), as well as conical spiniform process on inner proximal margin (arrowed in Fig. 3A). Fifth segment carrying setal elements Vv, Vd, Vm, 61, 2, aesthetasc 6aes, plus only 2 unbranched setae of “b” group (b6 and b3) on outer margin, as well as distal spiniform process at insertion of elements 61, 2, and 6aes (Figs. 2B, 3C). First pedigerous thoracic somite incorporated into cephalothorax, succeeding 3 free pedigerous somites each bearing pair of biramous swimming legs, all 3 together accounting for 31.3% of total body length (Fig. 1A). Endopodites and exopodites of swimming legs 1-4 unequal (exopods longer), triarticulate, and with same setal armature in each leg, except exopod of leg 1 with one fewer seta on the distal segment (Fig. 4D).
Armature formula of swimming legs: leg 1: basis 1-0, endopodite 0-1; 0-1; 1-2-2, exopodite I-1; 0-1; I-2-2; legs 2-4: basis 1-0, endopodite 0-1; 0-1; 1-2-2, exopodite I-1; 0-1; I-2-3. Coxae of legs 1-4 unarmed; each pair medially joined by subrectangular intercoxal sclerite about 1.3 times as long as broad with curved distal margin; anterior surface of sclerites 2-4 ornamented with rows of minute hyaline spinules. Basis separated from coxa posteriorly by diagonal articulation, lacking usual basipodal outer seta in legs 2-4. Outer distal corner of first and third exopodal segments of swimming legs 1-4 each with short, slender spiniform element about 1/3 as long as its segment. All natatory setae lightly and biserially plumose except for spiniform seta on outer distal corner of third exopodal segments, this being lightly setulate along inner side and bearing continuous row of small denticles along outer margin (Fig. 4D). Fifth legs medially conjoined, arising ventrally from posterior margin of fifth pedigerous somite (Fig. 4A), each being represented by elongate bilobed structure with its outer (exopodal) lobe armed with 2 subequally long apical setae. Unarmed and smooth inner (endopodal) lobe arising from proximal inner margin of outer lobe, almost reaching distal tip of latter. Urosome short (length = 389 µm long), accounting for 13.1% of total body length and consisting of fifth pedigerous somite, genital double-somite, 2 free abdominal somites, and caudal rami (Figs. 1C, 4A). Genital double somite representing 28.8% of length of urosome. Preanal somite about half as long as anal somite (Fig. 4A). Medial ventral surface of genital double-somite moderately swollen, bearing basally conjoined and posteriorly directed ovigerous spines (Fig. 4A). These spines (os in Figs. 1D, 4A) relatively short, corresponding to 21% of total body length and reaching to mid-length of caudal setae, each narrowing in its distal half to thin, seemingly socketed, seta-like section (Figs. 1D, 4A). Caudal rami subrectangular, 1.25 times as long as wide, moderately divergent, bearing 3 strong and subequally long terminal setae, as usual in genus (Figs. 1D, 4A), among 6 setal elements in all (I-VI), with innermost seta (VI) being shortest and proximal outer seta (I) being longest (Fig. 4A).
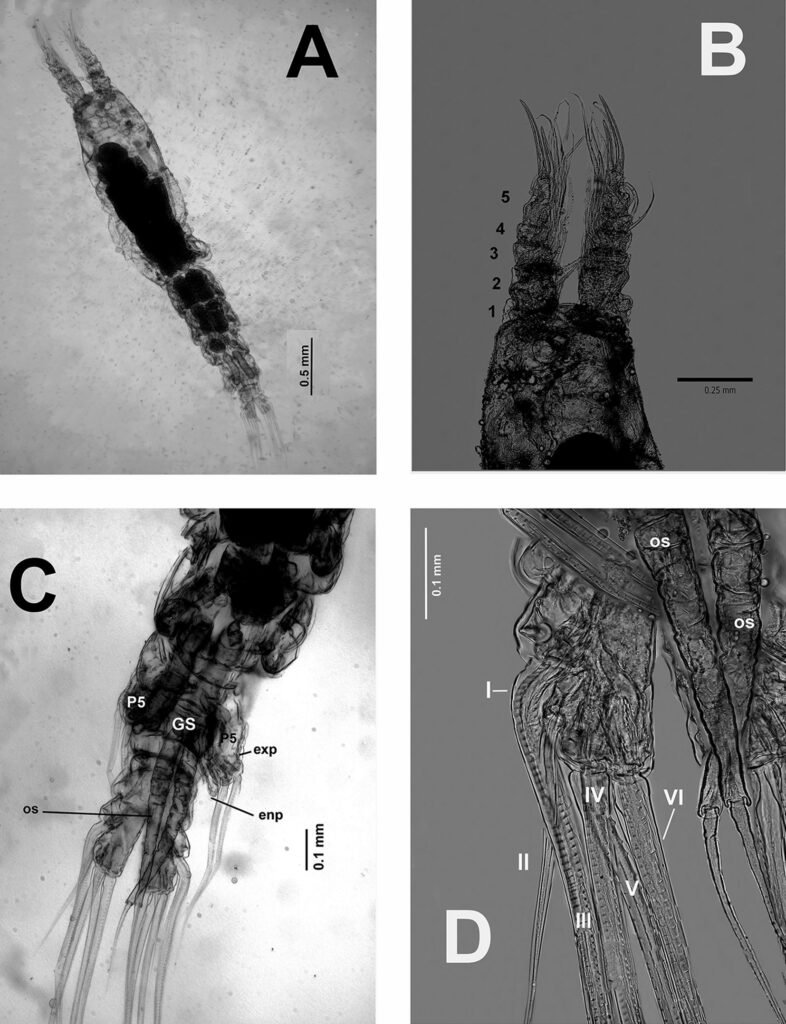
Figure 1. Monstrilla hendrickxi sp. n., from the Gulf of California, holotype female, digital photos. A, Habitus, dorsal view; B, anterior half of cephalothorax with 5- segmented antennules (1-5), ventral view; C, urosome, ventral view, showing ovigerous spines (os) arising from genital double-somite, fifth legs (P5) with 2 distal setae (1, 2), and caudal setae I-VI; D, distal part of ovigerous spines and caudal rami showing setae I-VI, ventral view.

Figure 2. Monstrilla hendrickxi sp. n., from the Gulf of California, holotype female, digital photos. A, Anterior part of cephalothorax showing weakly produced forehead and lack of eyes; B, antennule showing dorsal segmentation; C, fifth leg (P5) showing exopodal (exp) and endopodal (enp) lobes, ventral view; D, apical elements (61, 62, and 6aes) of distal antennular segment.

Figure 3. Monstrilla hendrickxi sp. n., from the Gulf of California, holotype female. A, Antennule showing setation labelled in accordance with Grygier and Ohtsuka’s (1995) nomenclature, ventral view; B, similarly labelled setation of second antennular segment, ventral view, also showing segment’s inner distal conical process (arrow); C, distal (fifth) segment of antennule, ventral view, showing similarly labelled setation and distal process (arrow); D, anterior third of cephalothorax, ventral view, showing integumental ornamentation, including nipple-like processes (nlp), anterior pore cluster (apc), and preoral pores (pp). Scales A-D = 100 µm.

Figure 4. Monstrilla hendrickxi sp. n., from the Gulf of California, holotype female. A, Urosome, ventral view, showing bilobed fifth leg with exopodal (exp) and endopodal (enp) lobes, ovigerous spines (os), and caudal rami setation (I-VI); B, anterior half of cephalothorax, ventral view, showing unlayed internal egg mass (em), oral cone (oc), and nipple-like processes (nlp); C, antennule segments 1-3 (S1-S3), ventral view, showing setation labelled in accordance with Grygier and Ohtsuka’s (1995) nomenclature, including modified setae IIIv and IIId; D, right leg 1, semi-lateral view, showing setation including basipodal seta (bs) and exopodal (exp) and endopodal (enp) rami. Scales A-D = 100 µm.
Taxonomic summary
Type locality. Southern Gulf of California, Mexico (25°53’15” N, 110°10’08” W). Sampling depth between 710 and 750 m.
Material examined. One subadult female (holotype), partially dissected and mounted on 2 semi-permanent glycerin slides sealed with acrylic nail varnish (ECO-CH-Z 11860). Epibenthic sledge, TALUD XVI B cruise, southern Gulf of California, Mexico, 31 May 2014.
Etymology. The specific name, a masculine genitive eponym, honors Dr. Michel E. Hendrickx (ICMyL-UNAM) for his sustained efforts and achievements in exploring the crustacean fauna of the Gulf of California and the Mexican Pacific.
Male. Unknown.
Host. Unknown.
Remarks
The sampling gear used to collect this specimen was an epibenthic sledge, not a plankton net; the latter is more efficient for capturing planktonic adult monstrilloids in surface waters. Epi-mesopelagic monstrilloids collected by sledge type gears have been reported from depths of 118 and 302 m in the North Atlantic (Suárez-Morales & Mercado-Salas, 2023), but those individuals were badly damaged even though the sledge had an attached plankton collector (Brandt et al., 2014). The specimen recovered from the TALUD XVI-B sledge sample was in reasonably good condition for taxonomic study.
The present subadult female monstrilloid from the Gulf of California can be readily assigned to the genus Monstrilla by its possession of the diagnostic generic features for females, including the presence of 2 somites between the genital double-somite and the anal somite, 6 caudal setae, and the oral cone’s location ventrally at nearly mid-length of the cephalothorax (Isaac, 1975; Suárez-Morales, 1994a). Among the species-level diagnostic features of M. hendrickxi sp. n., the most useful for comparison among congeneric species are the antennular structure and armature, the shape of leg 5, and the absence of eyes. These will be considered in sequence below.
The specifically distinctive characters of M. hendrickxi sp. n. include: 1) eyes and eye-related structures absent; 2) antennules relatively short, robust, representing nearly 33% of cephalothorax length, with segments 2-5 partly fused; 3) segments 2-5 furnished with modified setae or strong spiniform processes, 4) fifth antennulary segment with remarkably long, thick apical elements; 5) fifth leg bilobed, with digitiform endopodal lobe unarmed, exopodal lobe with 2 terminal setae; 6) 6 caudal setae, innermost seta (VI) being shortest, proximal outer seta (I) longest. The finding of subadult monstrillids in the plankton is not unusual and some species have been described from these individuals, including the first described monstrilloid copepod, Thaumaleus typica (Krøyer, 1842) (Grygier, 1994), Monstrilla capitellicola Hartman (1961), M. elongata Suárez-Morales, 1994a, and Monstrilla sp. from Hawaii (Suárez-Morales et al., 2014).
Partial or complete fusion of antennular segments 2-5 is found in several other species of Monstrilla, including M. ilhoii Lee & Chang, 2016, M. mariaeugeniae Suárez-Morales & Islas-Landeros, 1993, M. satchmoi Suárez-Morales & Dias, 2001, M. grandis Giesbrecht, 1891, M. gracilicauda Giesbrecht, 1893, and M. elongata Suárez-Morales, 1994a. None of these species displays the remarkable development of apical elements 61, 62, and 6aes observed in M. hendrickxi sp. n. The only available illustration of an antennule of M. nichollsi Davis, 1949 (= C. helgolandica) (Suárez-Morales pers. obs.) (cf. Nicholls, 1944: fig. 26, as Monstrilla sp.) shows a very long apical element on its fifth segment, which is probably identifiable as the aesthetasc 6aes. Elsewhere on the antennule, no congeneric species has modified setal elements IIId and IIIv on segment 3 or large, spiniform or conical processes on segments 2, 4, and 5 like those described in the new species.
Only a few known species originally described as Monstrilla possess a bilobed fifth leg with 2 setae on the outer (exopodal) lobe, 2 of them have been transferred to the genus Caromiobenella: C. helgolandica (Claus, 1863) and C. hamatapex (Grygier & Ohtsuka, 1995); the other species of Monstrilla sharing this character are M. mariaeugeniae, M. capitellicola, and M. leucopis Sars, 1921. Also, both Monstrilla sp. from Hawaii and M. capitellicola from Southern California (Hartman, 1961), likely represented by subadult individuals, also show only 2 setae on the outer lobe, a character conserved through the copepodiid stages CIII-V (Suárez-Morales et al., 2014). The new species M. hendrickxi differs from C. helgolandica, C. hamatapex, M. capitellicola, and M. leucopis, by its possession of a long, digitiform endopodal lobe, which is absent in these 4 species (Chang, 2014; Grygier & Ohtsuka, 1995; Sars, 1921; Zavarzin & Suárez-Morales, 2024). The corresponding endopodal lobe is clearly shorter in M. capitellicola (Hartman, 1961) than in the new species. Monstrilla wandelii Stephensen, 1913, and M. mariaeugeniae both exhibit a small, unarmed subtriangular endopodal lobe (Nicholls, 1944, fig 26; Park, 1967; Suárez-Morales & Islas-Landeros, 1993), which clearly differs from the elongate, digitiform endopodal lobe observed in M. hendrickxi sp. n.. Monstrilla nichollsi, a synonym of C. helgolandica (Suárez-Morales pers. obs.), was named by Davis (1949) based solely on Nicholl’s (1944, fig. 26) illustration of the fifth leg, thus allowing us to add C. helgolandica to the group of monstrillid species with 2 exopodal setae on the fifth leg exopodal lobe. It should be noted that the armature of the fifth leg exopodal lobe is conservative during the immature stages including the preadult CV (Suárez-Morales et al., 2014); changes in this character at the final molt are unlikely.
Monstrilla hendrickxi sp. n. is the only monstrilloid copepod in which no trace of the naupliar eye is present, although weakly developed visual structures have been observed previously in deep-living species (Suárez-Morales & Mercado-Salas, 2023). This contrasts with the usually well-developed, highly pigmented, three-cup naupliar eyes of most known monstrilloids. Functional eyes are probably extremely important for planktonic adult monstrilloids, allowing them, for example, to migrate to different light conditions in the water column and favor their dispersal (Suárez-Morales, 2018; Suárez-Morales & Gasca, 1990). The weak eye development of deep-living monstrilloids is likely an adaptive consequence of their aphotic habitat.
Monstrilla hendrickxi sp. n. is the fifth species of the copepod order Monstrilloida recorded from the Gulf of California, after Monstrilla gibbosa Suárez-Morales & Palomares-García, 1995, Spinomonstrilla spinosa (Park, 1967) (originally reported as Monstrilla spinosa), Cymbasoma californiense Suárez-Morales & Palomares-García, 1999, and recently M. leucopis Sars, 1921 (Suárez-Morales, 2019; Suárez-Morales & Palomares-García, 1999; Suárez-Morales & Velázquez-Ornelas, 2023).
Acknowledgements
We thank Michel E. Hendrickx (ICMyL-UNAM) for kindly allowing us to examine this specimen. Ship time for the TALUD XVI-B cruise was provided by the Coordinación de la Investigación Científica, UNAM, and partly supported by Conacyt (project # 179467). We also thank to an anonymous reviewer for the corrections made to improve this article.
References
Brandt, A., Havermans, C., Janussen, D., Jörger, K. M., Meyer-Löbbecke, A., Schnurr, S. et al. (2014). Composition and abundance of epibenthic-sledge catches in the South Polar Front of the Atlantic. Deep-Sea Research II, 108, 69–75. Doi?
Claus, C.(1863). Die frei lebenden Copepoden mit besonderer Berücksichtigung der Fauna Deutschlands, der Nordsee und des Mittelmeeres. Leipzig: Verlag von Wilhelm Engelmann.
Chang, C. Y. (2014). Two new records of monstrilloid copepods (Crustacea) from Korea. Animal Systematics, Evolution and Diversity, 30, 206–214. https://doi.org/10.5635/ASED.
2014.30.3.206
Cruz-Lopes da Rosa, J., Dias, C. O., Suárez-Morales, E., Weber, L. I., & Gomes-Fischer, L. (2021). Record of Caromiobenella (Copepoda, Monstrilloida) in Brazil and discovery of the male of C. brasiliensis: morphological and molecular evidence. Diversity, 13, 241. https://doi.org/
10.3390/d13060241
Dana, J. D. (1849). Conspectus crustaceorum, quae in orbis terrarum circumnavigatione, Carolo Wilkes, e classe Reipublicae foederatae duce, lexit et descripsit Jacobus D. Dana. Pars II. Proceedings of the American Academy of Arts and Sciences, 2, 9–61.
Davis, C. C. (1949). A preliminary revision of the Monstrilloida, with descriptions of two new species. Transactions of the American Microscopical Society, 68, 245–255. https://doi.org/10.2307/3223221
Giesbrecht, W. (1891). Elenco dei Copepodi pescati dalla R. Corvetta ‘Vettor Pisani’ secondo la loro distribuzione geografica. Atti della Accademia Nazionale dei Lincei, Classe di Scienze Fisiche Matematiche e Naturali Rendiconti, 4, 276–282.
Giesbrecht, W. (1893). Systematik und Faunistik der pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel und der angrenzenden Meeres-Abschnitte, 19, 1–831.
Grygier, M. J. (1994) [dated 1993]. Identity of Thaumatoessa (= Thaumaleus) typica Krøyer, the first described monstrilloid copepod. Sarsia, 78, 235–242. https://doi.org/10.1080/00364827.1993.10413537
Grygier, M. J., & Ohtsuka, S. (1995). SEM observation of the nauplius of Monstrilla hamatapex, new species, from Japan and an example of upgraded descriptive standards for monstrilloid copepods. Journal of Crustacean Biology, 15, 703–719. https://doi.org/10.1163/193724095X00118
Grygier, M. J., & Ohtsuka, S. (2008). A new genus of monstrilloid copepods (Crustacea) with anteriorly pointing ovigerous spines and related adaptations for subthoracic brooding. Zoological Journal of the Linnean Society, 152, 459–506. https://doi.org/10.1111/j.1096-3642.2007.00381.x
Hartman, O. (1961). A new monstrillid copepod parasitic in capitellid polychaetes in southern California. Zoologischer Anzeiger, 167, 325–334.
Huys, R., & Boxshall, G. A. (1991). Copepod evolution. London: Ray Society.
Huys, R., Llewellyn-Hughes, J., Conroy-Dalton, S., Olson, P. D., Spinks, J. N., & Johnston, D. A. (2007). Extraordinary host switching in siphonostomatoid copepods and the demise of the Monstrilloida: Integrating molecular data, ontogeny and antennulary morphology. Molecular Phylogenetics
and Evolution, 43, 368–378. https://doi.org/10.1016/j.ympev.
2007.02.004
Isaac, M. J. (1975). Copepoda, sub-order: Monstrilloida. Fiches d’Identification du Zooplancton, 144/145, 1–10.
Jeon, D., Lee, W., & Soh, H. Y. (2018). New genus and two new species of monstrilloid copepods (Copepoda: Monstrillidae): integrating morphological, molecular phylogenetic, and ecological evidence. Journal of Crustacean Biology, 38, 45–65. https://doi.org/10.1093 /jcbiol/rux095
Jeon, D., Lim, D., Lee, W., & Soh, H. Y. (2016). First use of molecular evidence to match sexes in the Monstrilloida (Crustacea: Copepoda), and taxonomic implications of the newly recognized and described partly Maemonstrilla-like females of Monstrillopsis longilobata Lee, Kim & Chang, 2016. Peer J, 13,e4938. https://doi.org/10.7717/peerj.4938
Krøyer, H. (1842). Crustacés. In P. E. Gaimard (Ed.), Atlas de Zoologie. Voyages de la Commission Scientifique du Nord en Scandinavie, en Laponie au Spitzberg et aux Feröe pendant les Anneés 1838, 1839 et 1840 sur la Corvette La Recherche, Commandée par M. Fabvre (pl. 41–43). Arthus Bertrand, Paris.
Lee, J., & Chang, C. Y. (2016). A new species of Monstrilla Dana, 1849 (Copepoda: Monstrilloida: Monstrillidae) from Korea, including a key to species from the North-west Pacific. Zootaxa, 4174, 396–409. https://doi.org/10.11646/zootaxa.4174.1.24
Nicholls, A. G. (1944). Littoral Copepoda from South Australia (II) Calanoida, Cyclopoida, Notodelphyoida, Monstrilloida, and Caligoida. Records of the South Australian Museum, 8, 1–62.
Park, T. S. (1967). Two unreported species and one new species of Monstrilla (Copepoda: Monstrilloida) from the Strait of Georgia. Transactions of the American Microscopical Society, 86, 144–152.
Razouls, C., Desreumaux, N., Kouwenberg, J., & de Bovée, F. (2023). Biodiversity of marine planktonic copepods (morphology, geographic distribution, and biological data [2005-2023]). Sorbonne University, CNRS. http://copepodes.obs-banyuls.fr/en [Accessed April 28, 2022]
Sale, P. F, McWilliams, P. S., & Anderson, D. T. (1996). Composition of the near-reef zooplankton at Heron Reef, Great Barrier Reef. Marine Biology, 34, 59–66. https://doi.org/10.1007/BF00390788
Sars, G. O. (1901). An account of the Crustacea of Norway with short descriptions and figures of all the species Vol. IV. Copepoda Calanoida. Bergen: The Bergen Museum.
Sars, G. O. (1921). An account of the Crustacea of Norway with short descriptions and figures of all the species, Vol. VIII. Copepoda Monstrilloida & Notodelphyoida. Bergen: The Bergen Museum.
Stephensen, K. (1913) Account of the Crustacea and the Pycnogonida collected by Dr. V. Nordmann in the summer of 1911 from northern Stromfjord and Giesecke Lake in West Greenland. Meddeleser om Groenland, 51, 55–77.
Suárez-Morales, E. (1994a). Monstrilla elongata, a new monstrilloid copepod (Crustacea: Copepoda: Monstrilloida) from a reef lagoon of the Caribbean coast of Mexico. Proceedings of the Biological Society of Washington, 107, 262–267.
Suárez-Morales, E. (1994b). Thaumaleus quintanarooensis, a new monstrilloid copepod from the Mexican coasts of the Caribbean Sea. Bulletin of Marine Science, 54, 381–384.
Suárez-Morales, E. (2001). An aggregation of monstrilloid copepods in a western Caribbean reef area: ecological and conceptual implications. Crustaceana, 74, 689–696. https://doi.org/10.1163/156854001750377966
Suárez-Morales, E. (2011). Diversity of the Monstrilloida (Crustacea: Copepoda). Plos One, 6, e22915. https://doi.org/10.1371/journal.pone.0022915
Suárez-Morales, E. (2018). Monstrilloid copepods: the best of three worlds. Bulletin of the Southern California Academy of Sciences, 107, 92–103. https://doi.org/10.22201/ib.20078706e.2020.91.3176
Suárez-Morales, E. (2019). A new genus of the Monstrilloida (Copepoda) with large rostral process and metasomal spines, and redescription of Monstrilla spinosa Park, 1967. Crustaceana, 92, 1099–1112. https://doi.org/10.1163/
15685403-00003925
Suárez-Morales, E., & Dias, C. O. (2001). Taxonomic report of some monstrilloids (Copepoda: Monstrilloida) from Brazil with description of four new species. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique Biologie, 71, 65–81.
Suárez-Morales, E., & Gasca, R. (1990). Variación dial del zooplancton asociado a las praderas de Thalassia testudinum en una laguna arrecifal del Caribe Mexicano. Universidad y Ciencia, 7, 57–64.
Suárez-Morales, E., Harris, L. H., Ferrari, F. D., & Gasca, R. (2014). Late postnaupliar development of Monstrilla sp. (Copepoda: Monstrilloida), a protelean endoparasite of benthic polychaetes. Invertebrate Reproduction & Development, 58, 60–73.
Suárez-Morales, E., & Islas-Landeros, M. E. (1993). A new species of Monstrilla (Copepoda: Monstrilloida) from a reef lagoon off the Mexican coast of the Caribbean Sea. Hydrobiologia, 271, 45–48.
Suárez-Morales, E., & McKinnon, A. D. (2014). The Australian Monstrilloida (Crustacea: Copepoda) I. Monstrillopsis Sars, Maemonstrilla Grygier & Ohtsuka, and Australomonstrillopsis gen. nov. Zootaxa, 3779, 301–340. https://doi.org/10.11646/zootaxa.3779.3.1
Suárez-Morales, E., & McKinnon, A. D., (2016). The Australian Monstrilloida (Crustacea: Copepoda) II. Cymbasoma Thompson, 1888. Zootaxa Monographs, 4102, 1–129. https://doi.org/10.11646/zootaxa.4102.1.1
Suárez-Morales, E., & Mercado-Salas, N. F. (2023). Two new species of Cymbasoma (Copepoda: Monstrilloida: Monstrillidae) from the North Atlantic. Journal of Natural History, 57, 1312–1330. https://doi.org/10.5852/ejt.
2024.917.2395
Suárez-Morales, E., Paiva Scardua, M., & Da Silva, P. M. (2010). Occurrence and histopathological effects of Monstrilla sp. (Copepoda: Monstrilloida) and other parasites in the brown mussel Perna perna from Brazil. Journal of the Marine Biological Association of the United Kingdom, 90, 953–958. https://doi.org/10.1017/S0025315409991391
Suárez-Morales, E., & Palomares-García, R. (1995). A new species of Monstrilla (Copepoda: Monstrilloida) from a coastal system of the Baja California Peninsula, Mexico. Journal of Plankton Research, 17, 745–752. https://doi.org/
10.1093/plankt/17.4.745
Suárez-Morales, E., & Palomares-García, R. (1999). Cymbasoma californiense, a new monstrilloid (Crustacea: Copepoda: Monstrilloida) from Baja California, Mexico. Proceedings of the Biological Society of Washington, 112, 189–198.
Suárez-Morales, E., Vásquez-Yeomans, L., & Santoya, L. (2020). A new species of the Cymbasoma longispinosum species-group(Copepoda, Monstrilloida, Monstrillidae) from Belize, western Caribbean Sea. Revista Mexicana de Biodiversidad, 91, e913176. https://doi.org/10.22201/ib.
20078706e.2020.91.3176
Suárez-Morales, E., & Velázquez-Ornelas, K. E. (2023). First record of Monstrilla leucopis G.O. Sars, 1921 (Copepoda: Monstrilloida: Monstrillidae) from the Eastern Pacific. Crustaceana, 96, 1183–1190. https://doi.org/10.1163/156854
03-bja10334
Thompson, I. C. (1888). Copepoda of Madeira and the Canary Islands, with descriptions of new genera and species. Zoological Journal of the Linnean Society, 20, 145–156. https://doi.org/10.1111/j.1096-3642.1888.tb01443.x
Walter, T. C., & Boxshall, G. A. (2022). World of copepods database Cymbasoma Thompson I.C., 1888. Accessed through: World Register of Marine Species (WoRMS)at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=119778 on 2022-04-23
Zavarzin, D., & Suárez-Morales, E. (2024). First record of Caromiobenella helgolandica (Claus, 1863) (Copepoda: Monstrilloida: Monstrillidae) from the Okhotsk Sea. Crustaceana, 97, 151–157. https://doi.org/10.1163/15685403-
bja10345