Taxonomía y Sistemática
Ecología

Evolución

Biogeografía

Notas científicas











Knowledge shortfalls and the effect of wildfireson biodiversity conservation in Guanajuato, Mexico
Tania Escalante a, *, Michelle Farfán b, Oscar Campos a, Leticia M. Ochoa-Ochoa c, Karen Flores-Quintal a, Diego R. García-Vélez a, Ana L. Medina-Bárcenas a, Fernando Saenz a
a Universidad Nacional Autónoma de México, Facultad de Ciencias, Grupo de Biogeografía de la Conservación, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Mexico City, Mexico
b Universidad de Guanajuato, Campus Guanajuato, División de Ingenierías, Departamento de Ingeniería Geomática e Hidráulica, Av. Juárez No. 77, Zona Centro, 36000 Guanajuato, Guanajuato, Mexico
c Universidad Nacional Autónoma de México, Facultad de Ciencias, Departamento de Biología Evolutiva, Museo de Zoología “Alfonso L. Herrera”, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Mexico City, Mexico
*Corresponding author: tescalante@ciencias.unam.mx (T. Escalante)
Received: 01 August 2023; accepted: 29 February 2024
Abstract
Knowledge of shortfalls could modify the geographic distribution patterns and limit the actions to conserve the biodiversity, even in the taxa best known. In addition, forest fires also could modify those patterns, but the potential effects of both factors have not been tested. Our aim was to analyze the effect of the Linnean and Wallacean shortfalls in the first evaluation of wildfire impacts on 22 amphibian and 13 mammal species distributed in Guanajuato, Mexico. We evaluated those shortfalls using the non-parametric estimator Chao2 and the Qs estimator and through maps of species richness patterns. To evaluate the effects of wildfires, we produced a fire recurrence map and quantified the burned area within species distributions and in 24 Protected Natural Areas (PNA) in the state. The Linnean shortfall showed some species missing to record in Guanajuato for both taxa, while the Wallacean shortfall showed poor quality of knowledge. Fire recurrence was high within 5 PNA. The richness patterns affected by fires covered nearly 17% of the surface of Guanajuato. Improving the knowledge of biogeographical patterns could provide better tools to stakeholders to decrease the negative impact of fires within PNA.
Keywords: Fire; Patterns; Priorities; Richness; Species distribution models
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Déficits de conocimiento y el efecto de los incendios forestales en la conservación de la biodiversidad en Guanajuato, México
Resumen
Los déficits en el conocimiento podrían modificar los patrones de distribución geográfica y limitar las acciones para conservar la biodiversidad, incluso en taxones bien conocidos. Además, los incendios forestales también pueden modificar esos patrones, pero los efectos potenciales de ambos no han sido probados. Nuestro objetivo fue analizar el efecto de los déficits Linneano y Wallaceano en la primera evaluación de los impactos de los incendios forestales en 22 especies de anfibios y 13 de mamíferos en Guanajuato, México. Evaluamos esos déficits utilizando los estimadores Chao2 y Qs y con mapas de riqueza de especies. Para evaluar los efectos de incendios forestales, elaboramos un mapa de recurrencia de incendios y cuantificamos el área quemada dentro de las distribuciones de las especies y en 24 áreas naturales protegidas (ANP). El déficit Linneano mostró que faltan algunas especies por registrar para ambos taxones, mientras que el déficit Wallaceano mostró una mala calidad de conocimiento. La recurrencia de incendios fue alta dentro de 5 ANP. Los patrones de riqueza afectados por los incendios cubrieron cerca de 17% de la superficie de Guanajuato. Mejorar el conocimiento de los patrones biogeográficos brindará mejores herramientas para disminuir el impacto de los incendios dentro de las ANP.
Palabras clave: Fuego; Patrones; Prioridades; Riqueza; Modelos de distribución de especies
Introduction
Terrestrial vertebrates are among the best known taxonomic groups, and it is assumed that their distributional areas and their biogeographic patterns are equally well known. However, there are shortfalls that could mask the distributional patterns and therefore, bias the actions to conserve those patterns. Linnean and Wallacean shortfalls affect our knowledge and lead to inaccurate representations of the species richness patterns in taxonomic groups that are presumed to be well known. The Linnean shortfall refers to the discrepancy between formally described species and the number of species that actually exist, while the Wallacean shortfall is the lack of knowledge about the geographical distribution of the species (Hortal et al., 2015; Lomolino, 2004). Both the Linnean and the Wallacean shortfalls can be difficult to evaluate, and they are rarely quantified in the literature prior to a conservation prioritization analysis. For example, to our knowledge, they have never been taken into account when analyzing the effects of wildfires on biodiversity.
Throughout the history of the Earth, fire has been a natural process that has driven the configuration of ecosystems and the maintenance of biodiversity around the world (He et al., 2019; Kelly et al., 2020). Indeed, there are many terrestrial ecosystems that are prone to fire and whose composition and structure are controlled by fire, leading to their classification as fire-adapted ecosystems (He et al., 2019; Schlisky et al., 2007). However, the forest fire regime has been altered by human dynamics associated with fire management and land use change at local and global scales (Chuvieco et al., 2008; Farfán et al., 2018; Martínez-Torres et al., 2015). Several authors agree that there is an increase in the occurrence of wildfires globally (Kelly et al., 2020). Places that did not burn naturally are now burning; examples include the tropical forests of Southeast Asia (Chisholm et al., 2016) and South America (Barlow et al., 2020) to the tundra of the Arctic Circle (Hu et al., 2015). Given the magnitude at which fires are occurring, it has even been proposed that the current era should be coined the Pyrocene, the “age of fire” (Pyne, 2021). This has led to the current situation in which frequency and intensity of forest fires pose a threat to biodiversity conservation worldwide and to human societies; this is due to the damage they cause but also by contributing to global warming.
In Mexico, the effects of wildfires on the fauna are poorly documented. Salazar et al. (2019) proposed a map (scale 1:50,000) of the severity of the fires in the state of Guanajuato for 2017, 2018 and 2019, by calculating the area of burned forest within each of 3 degrees of damage severity: low moderate, high moderate and high. They estimated the total burned area in Guanajuato at 8,460 ha in 2017; 19,589 ha in 2018; and 52,713 ha in 2019 (Salazar et al., 2019). Recently, Farfán et al. (2021) produced a map predicting the occurrence of fires in Guanajuato based on climatic variables under ENSO conditions using a spatial model. They observed that wildfires do not occur in random locations; rather, they are more likely to occur when fragmented forest is immersed in an agricultural matrix, as is frequently the case in the southern part of the state (Farfán et al., 2021).
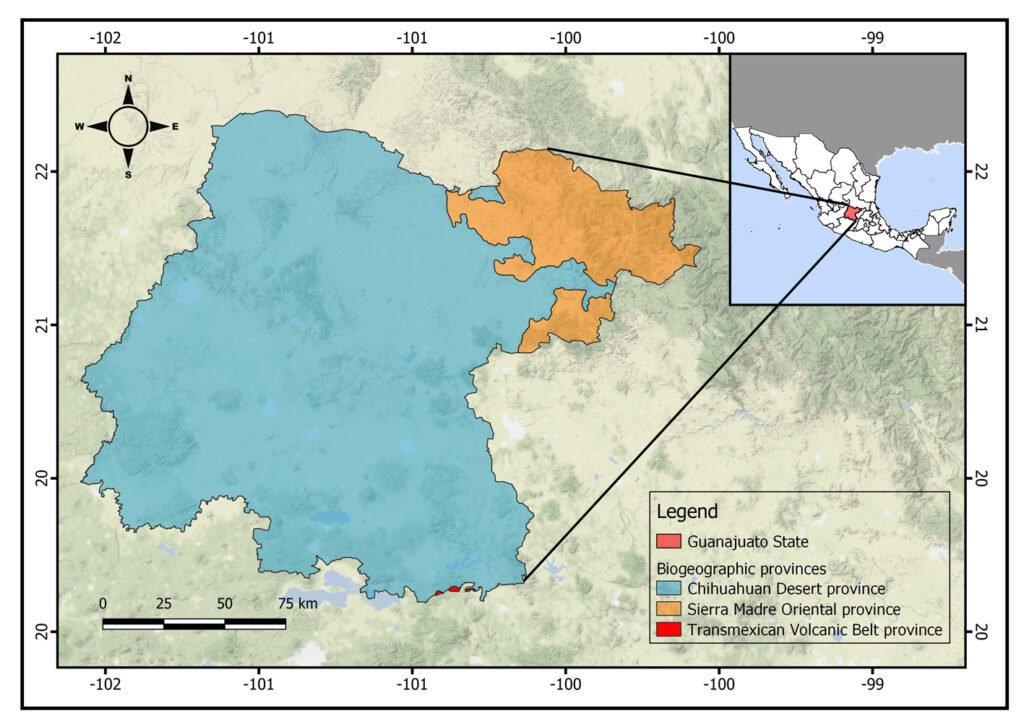
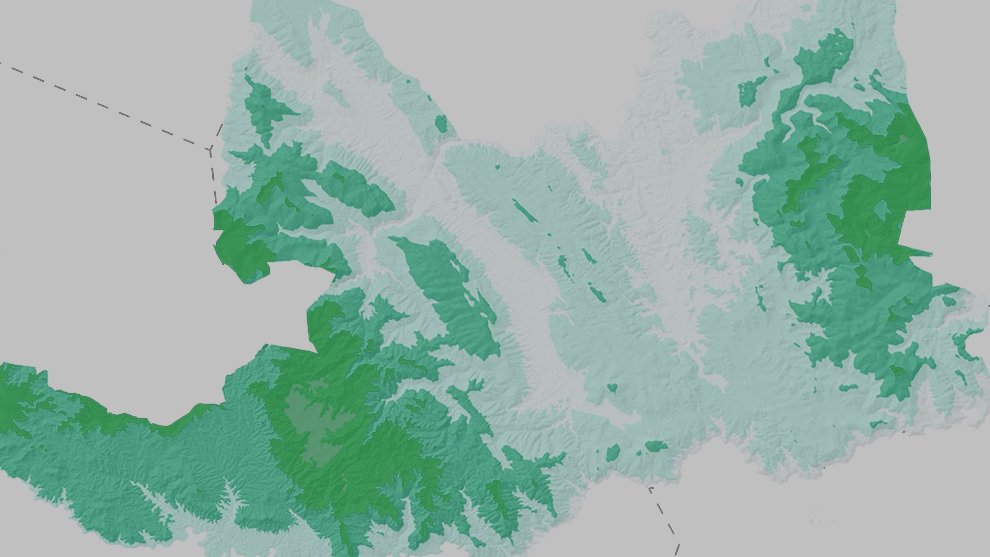
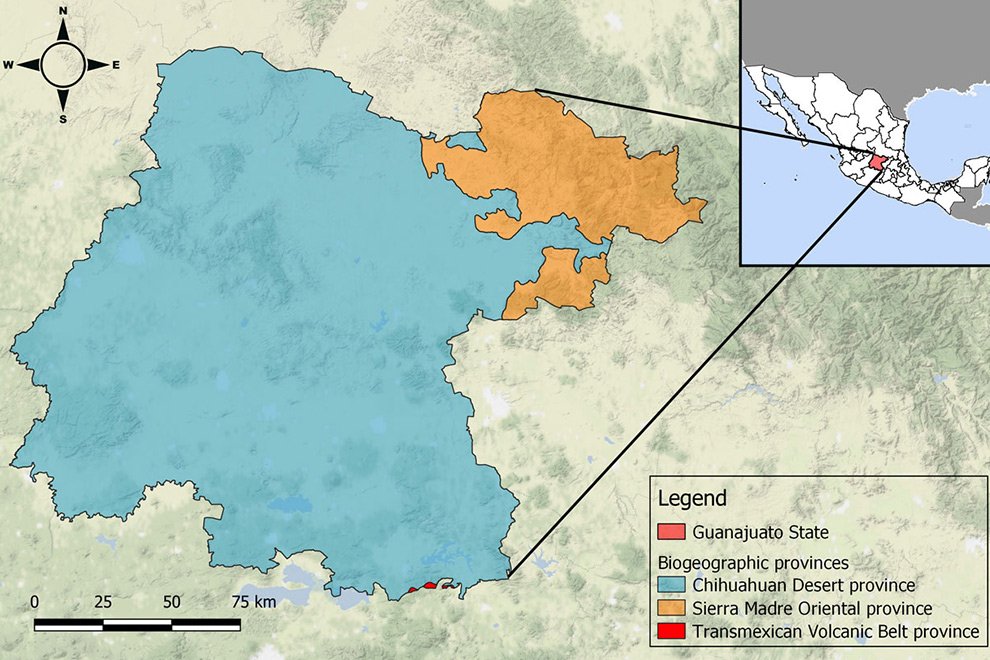
Figure 1. Location of the state of Guanajuato, Mexico, showing the biogeographic provinces.
Amphibians have been recognized as the most threatened terrestrial vertebrate class (Luedtke et al., 2023), and mammals could have significant declination in their populations due to fire, like in Australia (Geary et al., 2023). However, responses of animals to fire could be diverse because they are strongly related to their life-history traits (González et al., 2021). The responses of the amphibian species are variable and incompletely understood (Pilliod et al., 2003); while even among mammalian species, the effect of fire could be not consistent (González et al., 2021). Therefore, the effect of wildfires on distributional patterns of these taxa could be different.
In this study, our aim is to analyze the Linnean and Wallacean shortfalls in the context of the first evaluation of wildfire impacts on biodiversity. We use species of amphibians and mammals of Guanajuato, Mexico, as a study model and use the results to propose priority areas for conservation.
Material and methods
The state of Guanajuato is located in central Mexico, at 19°55’- 21°51’ N, 99°40’ – 102°06’ W. Most of the state is in the Chihuahuan Desert province, with a few areas in Sierra Madre Oriental and Transmexican Volcanic Belt provinces (Morrone et al., 2017; Fig. 1). Guajanuato has 24 Protected Natural Areas (PNA) mainly located in the southwest and center of the state. The largest of these areas is a biosphere reserve (Sierra Gorda), and the other PNA have a range of different levels of protection and activities allowed (SMAOT, 2022).
Although Guanajuato is not among the most biodiverse states in Mexico, it harbors a wide variety of ecosystems, from Pinus and Quercus forests to xerophytic scrubs (Conabio, 2012). Unfortunately, the extent of the agricultural and livestock areas, the high population density, the expansion of urban areas, and industrial activities have contributed to the destruction and disappearance of the original vegetation (Conabio, 2012).
Guanajuato harbors a total of 27 reported species of amphibians and 93 species of mammals, 8 and 25 of which, respectively, have been described as conservation priorities (DOF, 2010; Leyte-Manrique et al., 2022; Sánchez et al., 2016). Of all the species of amphibians and mammals inhabiting Guanajuato, we chose 22 species of amphibians and 13 species of mammals (Table 1) based on the following criteria: 1) valid nomenclature and at least one point record located in Guanajuato in the consulted databases (Flores-Villela & Ochoa-Ochoa, 2020; Escalante et al., 2018; GBIF.org, 2020a-ah); 2) geographic distribution mostly contained within Mexico; 3) at least 25 point records throughout the whole distribution in order to generate reliable species distribution models (SDM); and 4) considered conservation priorities.
In order to evaluate the Linnean and Wallacean shortfalls for those species, we searched the aforementioned databases for all valid point records in Guanajuato. These were initially overlapped to a grid of 0.25º latitude × 0.25º longitude, in QGIS v.3.16.16 (QGIS Development Team, 2020), which will be referred to hereafter as the “state scale”. We built a presence-absence matrix using the generated SDM.
To evaluate the Linnean shortfall based on point records, we quantified the observed richness (Sobs) as the recorded number of species of amphibians and mammals in each quadrat of 0.25º based on locality records. Then, we used the non-parametric estimator Chao2 in R (Kindt & Coe, 2005), to estimate the asymptotic richness of the incidence dataset (Gotelli & Colwell, 2011). The estimated richness Sest in Chao2 was obtained by the formula (Colwell & Coddington, 1994): Sest = Sobs + L2/(2M), where L = number of species that occur in only 1 quadrat, and M = number of species that occur in exactly 2 quadrats. Thus, this calculation provided a measure of how well the richness of each taxonomic group is known for those areas. We also performed the same analysis based on the species distribution models to explore how well the Linnean shortfall was corrected.
Table 1
List of species of amphibians and mammals in Guanajuato, Mexico, and data used in the analyses. Total records = number of point records after the nomenclatural and geographic validation (state scale). Records in Guanajuato = number of point records of each species into the geopolitical boundaries of the state of Guanajuato. Filtered records = subset of point records after the filter of 10 km applied to the total records. Records for modeling = subset of the filtered records used for model training. pROC = ROC partial of the best model.
| Species | Total records | Records in Guanajuato | Filtered records | Records for modeling | pROC |
| Amphibians | |||||
| Ambystoma velasci | 259 | 45 | 247 | 23 | 1.81 |
| Anaxyrus compactilis | 561 | 31 | 488 | 41 | 1.72 |
| Anaxyrus punctatus | 3,798 | 29 | 3,405 | 226 | 1.76 |
| Aquiloeurycea cephalica | 258 | 2 | 227 | 18 | 1.74 |
| Craugastor augusti | 599 | 16 | 508 | 54 | 1.58 |
| Dryophytes arenicolor | 2,514 | 138 | 2,269 | 161 | 1.81 |
| Dryophytes eximius | 820 | 19 | 761 | 72 | 1.75 |
| Eleutherodactylus guttilatus | 136 | 20 | 109 | 12 | 1.79 |
| Eleutherodactylus nitidus | 673 | 5 | 556 | 48 | 1.68 |
| Eleutherodactylus verrucipes | 237 | 2 | 167 | 13 | 1.52 |
| Hypopachus variolosus | 1,323 | 10 | 1,122 | 116 | 1.74 |
| Incilius nebulifer | 5,585 | 13 | 5,200 | 256 | 1.83 |
| Incilius occidentalis | 1,620 | 81 | 1,342 | 114 | 1.71 |
| Isthmura belli | 245 | 1 | 232 | 18 | 1.64 |
| Lithobates berlandieri | 3,747 | 51 | 3,401 | 247 | 1.56 |
| Lithobates megapoda | 127 | 9 | 101 | 12 | 1.65 |
| Lithobates montezumae | 696 | 94 | 598 | 51 | 1.75 |
| Lithobates neovolcanicus | 349 | 51 | 298 | 32 | 1.68 |
| Lithobates spectabilis | 544 | 3 | 416 | 40 | 1.64 |
| Rheohyla miotympanum | 383 | 1 | 329 | 27 | 1.60 |
| Smilisca baudinii | 4,381 | 2 | 3,590 | 277 | 1.68 |
| Spea multiplicata | 2,251 | 38 | 2,008 | 196 | 1.79 |
| Total | 31,090 | 661 | 27,374 | 2,054 | |
| Mammals | |||||
| Choeronycteris mexicana | 578 | 5 | 384 | 72 | 1.71 |
| Corynorhinus mexicanus | 204 | 4 | 149 | 28 | 1.79 |
| Dipodomys ornatus | 85 | 2 | 61 | 12 | 1.78 |
| Table 1. Continued | |||||
| Species | Total records | Records in Guanajuato | Filtered records | Records for modeling | pROC |
| Leptonycteris nivalis | 263 | 1 | 179 | 34 | 1.66 |
| Leptonycteris yerbabuenae | 575 | 9 | 370 | 70 | 1.75 |
| Lepus callotis | 199 | 6 | 150 | 28 | 1.75 |
| Peromyscus melanotis | 601 | 3 | 227 | 42 | 1.84 |
| Peromyscus difficilis | 897 | 10 | 453 | 85 | 1.69 |
| Peromyscus melanophrys | 594 | 25 | 372 | 70 | 1.59 |
| Rhogeessa alleni | 55 | 5 | 46 | 8 | 1.60 |
| Sciurus oculatus | 123 | 34 | 73 | 14 | 1.45 |
| Sigmodon leucotis | 103 | 9 | 72 | 14 | 1.50 |
| Sorex saussurei | 168 | 1 | 93 | 18 | 1.8 |
| Total | 4,445 | 114 | 2,629 | 495 |
To quantify the Wallacean shortfall, we used the Qs estimator (Murguía-Romero & Villaseñor, 2000), which is a measure of the quality of the records. QS can take values between ‘0’ and ‘1’ and is defined as (Murguía-Romero & Villaseñor, 2000): QS = F/[Sobs m/(1 – Es) – max (Sobs, m)], where F = the sum of frequencies of all classes multiplied by all classes (that is, the sum of all ‘1’ in the matrix); Es = measure of the proportion of the known richness related to the estimated richness; and m = the total number of quadrats. Murguía-Romero and Villaseñor (2000) characterized QS values above 80% as “very good”, values between 50% to 80% as “good” and less than 50% as “poor” data quality.
To compare the possible effect of the Wallacean shortfall in the biogeographic patterns, we performed a species distribution model (SDM) for each species in order to obtain a map of richness patterns for amphibians and mammals. Following the BAM diagram of Soberón and Peterson (2005), where the M corresponds to the region that is reachable by the species from established distributional areas in ecological time (Soberón & Peterson, 2005). The M for each species was obtained using the concept of extent of occurrence, defined as “the area contained within the shortest continuous imaginary boundary that can be drawn to encompass all the known, inferred or projected sites of present occurrence of a taxon, excluding cases of vagrancy” (IUCN, 2001). Therefore, we defined M as the area within a minimum convex hull polygon for each species constructed in QGIS v.3.16.16 (QGIS Development Team, 2020).
The M of each species was used to crop the 19 environmental layers of WorldClim 2 (Fick & Hijmans, 2017) and 3 topographic variables (slope, elevation and aspect; USGS, 2021) at ~ 1 km2 of resolution. The data points were filtered in Wallace software (Kass et al., 2018) to a distance of 10 km between points to reduce spatial biases, and retain useful information (Aiello-Lammens et al., 2015; Pearson et al., 2007). To avoid collinearity among the 22 variables for each species, we obtained the VIF (Mandeville, 2008; Montgomery & Peck, 1992), applying the packages usdm (Naimi et al., 2014) and rgdal (Bivand et al., 2015) in RStudio (RStudio Team, 2020).
The models were performed in the maximum entropy package kuenm (Cobos et al., 2019) in RStudio (RStudio Team, 2020). The occurrence dataset for each species was divided as follows: 75% of the points were used for training and 25% for testing; and a set of independent occurrences of 25% for a last evaluation; those datasets were built with the kuenm_occsplit function in kuenm. For the next step, we used the function kuenm_cal, using the feature classes: linear, quadratic and hinge; and the regularization multipliers 0.5, 1, 1.5, 2, 2.5, 3, 3.5 and 4. All models were evaluated with kuenm_ceval, calculating the ROC partial with E = 10 (Peterson et al., 2008), and Akaike criterion for small samples (AICc; Warren & Seifert, 2011). The final best model for each species was obtained on a clog-log scale using the pROC value in NicheToolbox (Osorio-Olvera et al., 2020).
To produce binary maps of geographic distribution area of each species, the final best model was reclassified using the “10th percentile training presence” threshold, and cropped to the political boundaries of Guanajuato.
Wildfires. We used hotspot data from the MODIS sensor at a resolution of 1 km2 for the years 2000 to 2021, downloaded from NASA Earth Data Cloud (2020). Each hotspot was overlapped to a net of ~ 1 km2 covering the state of Guanajuato in QGIS (QGIS Development Team, 2020), and we counted the number of hotspots in each square of the net. This area will be referred to as the “fine scale”.
In order to compare the number of fires in each square of the net with the scale of the models, we transformed this number between ‘0’ and ‘1’ through a min-max normalization (Farrús et al., 2007; Jain et al., 2005). This procedure was useful to evaluate the effect of the wildfires on the SDM of each species. All cartographic products were projected to UTM zone 14 north coordinates, which corresponds to the state of Guanajuato. To quantify the impact of the fires on species richness patterns, we rasterized the map of the number of fires, from which we produced a new map of kernel density with a radius of 3,000 m, using the software DINAMICA EGO (Ferreira et al., 2019). The map of kernel density was multiplied by the richness map for each taxonomic group. Finally, we quantified the burned area for the maps and for the Protected Natural Areas (SMAOT, 2022).
Results
Shortfalls. We obtained 31,090 records of amphibians considering the whole distribution for the 22 amphibian species, and 661 records within Guanajuato. For the 13 species of mammals, the number of records was 74 in Guanajuato and 2,629 in the whole distribution. The species with the most records was the coastal plain toad Incilius nebulifer (5,585 records) for the amphibians and the southern rock mouse Peromyscus difficilis (453 records; see Table 1) for the mammals. However, within Guanajuato, there were only 13 and 9 records for these species, respectively. The species with the highest number of point records within Guanajuato were the canyon tree frog Dryophytes arenicolor with 138 records, and the Peters’s squirrel Sciurus oculatus, with 34 records.
The richest 0.25º quadrat for amphibians had 11 species and the quadrat with the most mammals had 5 species, while the lowest number of species per quadrat was 1 for both taxa, although there were a few marginal quadrats without data for amphibians and some complete quadrats without data for mammals (~ 12). The maps of quadrats with the observed richness (Sobs in Chao2) for both groups are shown in the Supplementary material: figures 1S, 2S. The main results of the Chao2 estimator are shown in Table 2. For both groups, the number of observed species (Sobs) was lower than the expected number (Sest) for Guanajuato. In the case of the Chao2 estimated with species distribution models, the species richness was the same as the expected species.
Regarding the Wallacean shortfall, the Qs estimator had a value of 23% for the amphibians and 28% for mammals at the state scale. These values were categorized as “poor” quality data in both taxa, but Qs was worse in amphibians.
Table 2
Results of the recorded and estimated richness for the complete distributional data of amphibians and mammals of Guanajuato, Mexico based both on species richness and on species distribution models (SDM).
| Taxonomic group | Recorded richness (Sobs) | Estimated richness (Sest) | Standard deviation (SD) | Estimated richness with SDM (Sest) |
| Amphibians | 22 | 24.61 | 3.42 | 24 |
| Mammals | 13 | 14.94 | 3.64 | 14 |

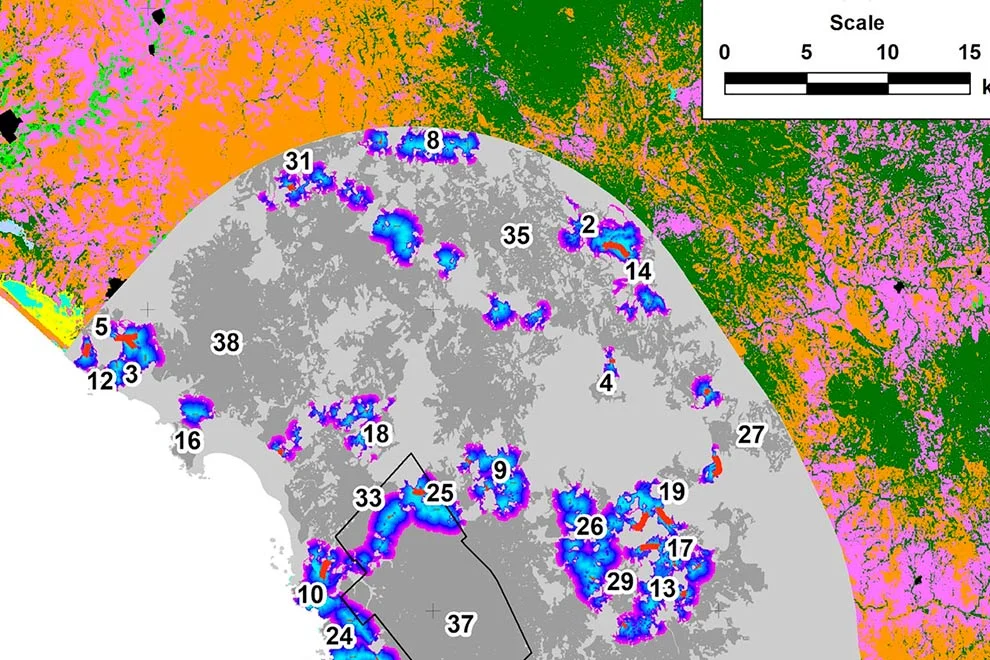
Figure 2. Richness pattern for 22 modeled species of amphibians and the Protected Natural Areas in Guanajuato, Mexico (black polygons): 1. Palenque, 2. Peña Alta, 3. Sierra de Pénjamo, 4. Sierra de los Agustinos, 5. Las Fuentes, 6. Sierra de Lobos, 7. Las Musas, 8. Lago Cráter La Joya, 9. Cerro de los Amoles, 10. Parque Metropolitano, 11. Cerro de Arandas, 12. Cuenca Alta del Río Temascatio, 13. Cerro del Cubilete, 14. Cerros El Culiacán y La Gavia, 15. Cuenca de La Esperanza, 16. Mega Parque de la Ciudad de Dolores Hidalgo, 17. Presa La Purísima y su Zona de Influencia, 18. Presa de Neutla y su Zona de Influencia, 19. Laguna de Yuriria y su Zona de Influencia, 20. Pinal de Zamorano, 21. Región Volcánica Siete Luminarias, 22. Sierra Gorda de Guanajuato, 23. Cuenca de la Soledad, 24. Presa de Silva y Áreas Aledañas.
Some results of the modeling process are shown in Table 1. The distribution models of amphibians predicted that the species Craugastor augusti, Dryophytes arenicolor, Dryophytes eximius, Incilius occidentalis, Lithobates montezumae, Lithobates neovolcanicus, and Spea multiplicata are distributed in more than 90% of the surface of Guanajuato. Meanwhile, the species Aquiloeurycea cephalica (31%), Smilisca baudini (25%), Lithobates berlandieri (19%), and Incilius nebulifer (3%) had the lowest proportion of distribution in Guanajuato. Regarding the mammals, Choeronycteris mexicana, Corynorhynus mexicanus, Leptonycteris nivalis, Lepus callotis, Peromyscus difficilis, Peromyscus melanophrys, and Peromyscus melanotis were the most widely distributed in Guanajuato (more than the 90% of the state is predicted as part of their distribution), while Sorex saussurei was the only species with a proportion less than 30%.
For the richness patterns, the pixels with highest number of species modeled to be present were similar between amphibians and mammals at the fine scale (Figs. 2, 3), showing a diagonal strip of high richness from the northwest to the southeast, which coincided with 14 PNA: Las Fuentes, Sierra de Lobos, Parque Metropolitano, Cuenca Alta del Río Temascatío, Cerro del Cubilete, Cuenca de La Esperanza, Presa de Neutla, Cuenca de la Soledad, Sierra de los Agustinos, Lago Cráter La Joya, Cerro de los Amoles, Cerros El Culiacán y La Gavia, Laguna de Yuriria and Región Volcánica Siete Luminarias. There were also other sites of high diversity, for example within the PNA of Sierra de Pénjamo and Sierra Gorda.
Wildfires. The maximum number of wildfires per quadrant at the fine scale was 6. To further explore the risk of wildfire recurrence, we built a risk map using the recurrence of fires in each square, with 3 classes: 1) low risk, for pixels with one fire during the analyzed period; 2) medium risk, for pixels with 2 or 3 fires; and 3) high risk, for pixels with 4, 5 or 6 fires (Fig. 4). A large proportion of quadrats with high recurrence of fires occurred outside PNA (for example at northern Guanajuato), but there were also some high risk zones within PNA, like Palenque, Peña Alta, Sierra de Pénjamo, Las Musas and Región Volcánica Siete Luminarias.

Figure 3. Richness pattern for 13 modeled species of mammals and the Protected Natural Areas in Guanajuato, Mexico (black polygons): 1. Palenque, 2. Peña Alta, 3. Sierra de Pénjamo, 4. Sierra de los Agustinos, 5. Las Fuentes, 6. Sierra de Lobos, 7. Las Musas, 8. Lago Cráter La Joya, 9. Cerro de los Amoles, 10. Parque Metropolitano, 11. Cerro de Arandas, 12. Cuenca Alta del Río Temascatio, 13. Cerro del Cubilete, 14. Cerros El Culiacán y La Gavia, 15. Cuenca de La Esperanza, 16. Mega Parque de la Ciudad de Dolores Hidalgo, 17. Presa La Purísima y su Zona de Influencia, 18. Presa de Neutla y su Zona de Influencia, 19. Laguna de Yuriria y su Zona de Influencia, 20. Pinal de Zamorano, 21. Región Volcánica Siete Luminarias, 22. Sierra Gorda de Guanajuato, 23. Cuenca de la Soledad, 24. Presa de Silva y Áreas Aledañas.
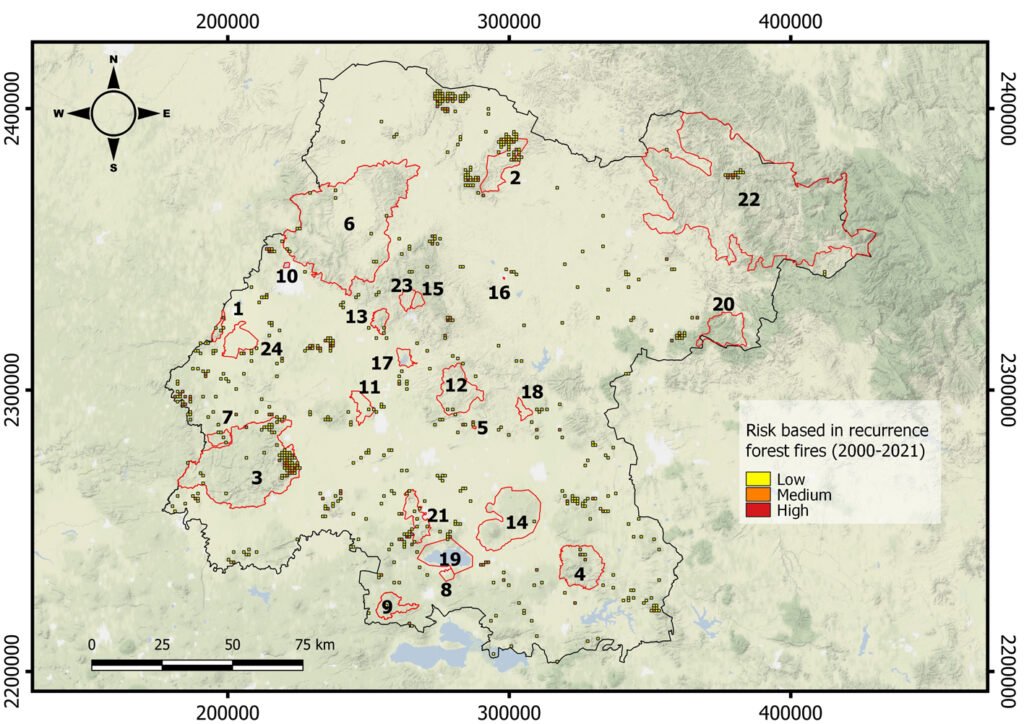
Figure 4. Map of fire risk in Guanajuato between the years 2000 and 2021, based on the recurrence of fires in a 1 km square, with 3 classes: (1) low risk, for squares with one fire during the analyzed period; (2) medium risk, for squares with 2 or 3 fires; and (3) high risk, for squares with 4, 5 or 6 fires. Red polygons represent the Protected Natural Areas.
Table 3
Potential distribution area predicted by the modeling for 22 amphibian and 13 mammal species and quantification of habitat lost due to wildfires relative to the total area of Guanajuato, Mexico (30,702 km2).
| Species | Surface of potential distribution area occupied in Guanajuato (km2) | Percentage of potential distribution area occupied (%) | Surface of potential distribution area affected by wildfires (km2) | Percentage of potential distribution area affected by wildfires (%) |
| Amphibians | ||||
| Ambystoma velasci | 22,351 | 73 | 3,620 | 16 |
| Anaxyrus compactilis | 26,061 | 85 | 4,948 | 19 |
| Anaxyrus punctatus | 18,241 | 59 | 2,440 | 13.3 |
| Aquiloeurycea cephalica | 9,653 | 31 | 1,246 | 12.9 |
| Craugastor augusti | 30,702 | 100 | 5,264 | 17.1 |
| Dryophytes arenicolor | 30,696 | 99.9 | 5,262 | 17.1 |
| Dryophytes eximius | 30,267 | 98.5 | 5,249 | 17.3 |
| Eleutherodactylus guttilatus | 21,636 | 70 | 2,088 | 14.2 |
| Eleutherodactylus nitidus | 22,888 | 75 | 4,663 | 20.3 |
| Eleutherodactylus verrucipes | 20,519 | 67 | 2,756 | 13.4 |
| Hypopachus variolosus | 19,076 | 62 | 3,951 | 20.7 |
| Incilius nebulifer | 873 | 3 | 55 | 6.3 |
| Incilius occidentalis | 30,546 | 99 | 5,257 | 17.2 |
| Isthmura bellii | 26,647 | 87 | 4,925 | 18.4 |
| Lithobates berlandieri | 5,772 | 19 | 614 | 10.6 |
| Lithobates megapoda | 20,866 | 68 | 4,422 | 21.1 |
| Lithobates montezumae | 29,957 | 97.5 | 5,242 | 17.4 |
| Lithobates neovolcanicus | 28,670 | 93 | 5,137 | 17.9 |
| Lithobates spectabilis | 24,821 | 81 | 3,893 | 15.6 |
| Rheohyla miotympanum | 18,056 | 59 | 3,516 | 19.4 |
| Smilisca baudinii | 7,631 | 25 | 1,579 | 20.6 |
| Spea multiplicata | 30,661 | 99.8 | 5,264 | 17.1 |
| Mammals | ||||
| Choeronycteris mexicana | 30,608 | 99.69 | 5,242 | 17.12 |
| Corynorhynus mexicanus | 30,608 | 99.69 | 5,242 | 17.12 |
| Dipodomys ornatus | 18,553 | 60.43 | 3,429 | 18.48 |
| Leptonycteris nivalis | 30,608 | 99.69 | 5,242 | 17.12 |
| Leptonycteris yerbabuenae | 24,955 | 81.28 | 4,474 | 17.93 |
| Lepus callotis | 28,469 | 92.73 | 5,025 | 17.65 |
| Peromyscus difficilis | 30,142 | 98.18 | 5,225 | 17.34 |
| Peromyscus melanophrys | 29,606 | 96.43 | 5,227 | 17.65 |
| Peromyscus melanotis | 29,231 | 95.21 | 5,183 | 17.73 |
| Rhogessa alleni | 17,604 | 57.34 | 3,353 | 19.05 |
| Sciurus oculatus | 14,564 | 47.44 | 1,923 | 13.20 |
| Sigmodon leucotis | 18,215 | 59.33 | 3,139 | 17.23 |
| Sorex saussurei | 4,151 | 13.52 | 756 | 18.22 |

Figure 5. Richness map of amphibians intersected with the kernel density of recurrence of the wildfires in Guanajuato, Mexico. Black polygons represent the Protected Natural Areas.
1. Palenque, 2. Peña Alta, 3. Sierra de Pénjamo, 4. Sierra de los Agustinos, 5. Las Fuentes, 6. Sierra de Lobos, 7. Las Musas, 8. Lago Cráter La Joya, 9. Cerro de los Amoles, 10. Parque Metropolitano, 11. Cerro de Arandas, 12. Cuenca Alta del Río Temascatio, 13. Cerro del Cubilete, 14. Cerros El Culiacán y La Gavia, 15. Cuenca de La Esperanza, 16. Mega Parque de la Ciudad de Dolores Hidalgo, 17. Presa La Purísima y su Zona de Influencia, 18. Presa de Neutla y su Zona de Influencia, 19. Laguna de Yuriria y su Zona de Influencia, 20. Pinal de Zamorano, 21. Región Volcánica Siete Luminarias, 22. Sierra Gorda de Guanajuato, 23. Cuenca de la Soledad, 24. Presa de Silva y Áreas Aledañas.
Respect to the temporal distribution of the recurrence of forest fires, for the period of time analyzed, the years 2017, 2019, and 2021 had the highest number of fires (72, 110 and 150, respectively). The map of kernel density is shown in Supplementary material: Figure 3S. The map was transformed to a binary map and overlapped with the patterns of richness of amphibians and mammals, to produce the maps in figures 5, 6.
The evaluation of the effects of the wildfires on the model of each species is shown in Table 3. The mean percentage of burned potential distribution area was 16.49 and 17.37 for amphibians and mammals, respectively. Some species’ distribution areas were more strongly affected by fires, such as Eleutherodactylus nitidus, Hypopachus variolosus, Lithobates megapode, and Smilisca baudinii, all of which were amphibians for which more than 20% of their distribution area had been burned. For mammals, the most affected species was Rhogessa alleni (19.05%), followed by Sorex saussurei (18.22%).
The area of Guanajuato affected by fires measured nearly 5,200 km2 (17%; Figs. 5, 6), with high diversity zones for amphibians located in the northwest, near Sierra de Lobos (PNA 6) and Peña Alta (PNA 2). Other important affected areas for amphibians coincided with high recurrence of wildfires in the southern of Guanajuato in Región Volcánica Siete Luminarias (PNA 21), Sierra de los Agustinos (PNA 4) and Cerro de los Amoles (PNA 9). For mammals, some of the most strongly affected areas coincided with those of the amphibians (e.g., within Cerro de los Amoles; PNA 9), but there were also areas that were unique to mammals (Fig. 6). For example, there were areas of high mammal richness with wildfires in central Guanajuato, which did not coincide with any PNA, as well as southeastern areas in Sierra de los Agustinos (PNA 4). Fortunately, areas with high richness for both amphibians and mammals were not affected by fires, like Sierra de Lobos (PNA 6), Cuenca de la Esperanza (PNA 15), and Cuenca de la Soledad (PNA 23).
Discussion
Linnean shortfalls in the state of Guanajuato could have medium effects because the estimator predicted at least 2 additional species for each taxon relative to total currently known. This suggests that the current species inventories are not yet complete. This finding does not dismiss possible shortfalls at more detailed scales, because the number of records within the state of Guanajuato is very low, with an average of 30 per each species of amphibian and only 6 of each species of mammal. It would therefore be informative to carry out more specific analyses within the quadrats where 0 or 1 species were recorded. Increased collection effort in the field could improve the problems of undersampling, since the number of total data points in Guanajuato is very low for some species (v. gr. Aquiloeurycea cephalica with 2 records, and Leptonycteris nivalis with 1 record; Table 1). It is interesting to highlight that when performing the analyses with SDM, the Linnean shortfall is apparently corrected (Table 2). However, these results should be taken with caution, because it is possible that there are commission errors in models or that those areas actually correspond to sister species (Rodrigues et al., 2019; Acevedo et al., 2014).

Figure 6. Richness map of mammals intersected with the kernel density of recurrence of the wildfires in Guanajuato, Mexico. Black polygons represent the Protected Natural Areas.
1. Palenque, 2. Peña Alta, 3. Sierra de Pénjamo, 4. Sierra de los Agustinos, 5. Las Fuentes , 6. Sierra de Lobos, 7. Las Musas, 8. Lago Cráter La Joya, 9. Cerro de los Amoles, 10. Parque Metropolitano, 11. Cerro de Arandas, 12. Cuenca Alta del Río Temascatio, 13. Cerro del Cubilete, 14. Cerros El Culiacán y La Gavia, 15. Cuenca de La Esperanza, 16. Mega Parque de la Ciudad de Dolores Hidalgo, 17. Presa La Purísima y su Zona de Influencia, 18. Presa de Neutla y su Zona de Influencia, 19. Laguna de Yuriria y su Zona de Influencia, 20. Pinal de Zamorano, 21. Región Volcánica Siete Luminarias, 22. Sierra Gorda de Guanajuato, 23. Cuenca de la Soledad, 24. Presa de Silva y Áreas Aledañas.
On the other hand, the Wallacean shortfall was highly relevant for both amphibians and mammals, showing poor quality. Murguía-Romero and Villaseñor (2000) suggested that the quality of the records is related to the geographical resolution of the biogeographical analysis. In future analysis within the state of Guanajuato and using smaller quadrats (for example, close in size to the pixels of our models), the Wallacean shortfall could strongly affect the observed data, reaching very poor data quality. Thus, it seems to be the more important shortfall for these vertebrate species.
For our 25 species, probably the effect of the Linnean shortfall has a less dramatic effect than the Wallacean shortfall. There are multiple potential explanations for these shortfalls, including low intensity and spatial variation of sampling, which can directly affect biodiversity estimators like species richness (Oliveira et al., 2016). Continuing the study of these shortfall will be important for the correct implementation of conservation strategies, for example with other methods including correlations using the sampling effort (Oliveira et al., 2016), rate of descriptions and number of taxonomists (Joppa et al., 2011), many different algorithms of species distribution modeling and maps of ignorance (Oliveira et al., 2016; Rocchini et al., 2011; Tessarolo et al., 2021), among others.
In spite of the extant Wallacean shortfalls, the richness patterns for both taxa were partially recognized; specially, the recorded most richness quadrats at 0.25º also showed the modeled pattern for amphibians. For mammals, the highest richness area near to Cuenca de la Esperanza was identified also for a quadrat of 0.25º, coinciding with the models. Amphibians and mammals shared many (though not all) areas of high richness, generally following a northwest-southeast diagonal across the state. The partial similarity in those richness patterns could be useful in Systematic Conservation Planning, because both taxa could represent each other as good surrogates (Escalante et al., 2020).
In general, amphibians and mammals are over-represented taxa in databases, although amphibians are less represented than mammals (Troudet et al., 2017). Few articles have quantified the Linnean and Wallacean shortfalls prior to biogeographic analysis. In particular, Oliveira et al.(2016) suggested that terrestrial vertebrates have similar biases compared with some taxa of arthropods, contradicting the statement that terrestrial vertebrates are better suited for biogeographic and conservation studies. In some places, such as the state of Guanajuato, amphibians and mammals could have similar Linnean shortfalls, but differ in the severity of their Wallacean shortfalls, which could modify the biogeographic patterns identified.
The relevance of including shortfall analysis in biogeographical studies, mainly in those related to species conservation, lies in the fact that the Linnean and Wallacean shortfalls strongly influence the possible results, since the data on the identity and distribution of the species are crucial to identify patterns in biodiversity, as well as the processes that modify those patterns (Hortal et al., 2015). In particular, Wallacean shortfalls can also alter estimates of threatened conservation status, since range size is regularly used in conservation (Hortal et al., 2015). Species with small ranges have higher priority in many international and national standards (DOF, 2010; IUCN, 2012). Therefore, Wallacean shortfalls could lead to some taxa and areas being disproportionately prioritized over others because their distribution areas have been erroneously underestimated (Riddle et al., 2011). In addition, other shortfalls that potentially can affect the biogeographic patterns should be investigated, like Darwinian shortfall (Diniz-Filho et al., 2013, 2023), and even distinct categories of Linnean shortfalls (Vergara-Asenjo et al., 2023).
As we expected, wildfires affected all species, but in different ways. For amphibians, the species Smilisca baudinii, in addition to having a small distribution area in Guanajuato compared to the rest of the species, is one of the most affected by wildfires occurrences. These observations may suggest that Smilisca baudinii should be considered a priority species for conservation in the state of Guanajuato. Furthermore, we also highlight Lithobates megapoda, which is listed in the 2019 update of the NOM-059-SEMARNAT-2010 (DOF, 2019) as a species under special protection and described as sensitive to habitat degradation (Santos-Barrera & Flores-Villela, 2004).
On the other hand, in the northeastern part of the state, the effects seem to be minimal compared to the south and southwest, but it is important to remember that species with specific habitat requirements such as Incilius nebulifer, Lithobates berlandieri, and Smilisca baudinii are distributed in this area. Therefore, attention should be paid to investigating the sources of ignition present at this area in order to prevent future wildfires, since the loss of habitat could result in the disappearance of these species. Finally, in accordance with Clivillé et al.(1997), who describe the effect of fires on amphibians from 3 points of view (habitat, species and individual), our study only focuses on the effect of these events on the habitat. Thus, the effects on the distribution of the selected species can be interpreted as loss of habitat and vegetation cover, trophic resources and humidity due to wildfires, which are determining characteristics for the presence of amphibians and their reproduction (Clivillé et al., 1997).
For the case of mammals, Sorex saussurei (the Saussure’s shrew) is the species with the narrowest geographic distribution in Guanajuato. This shrew is only distributed in Mexico and Guatemala, and even though it is considered as least concern on the Red List of Threatened Species (IUCN, 2017), some populations in Mexico have been categorized as threatened and under special protection (DOF, 2019). Secondly, Peters’s squirrel Sciurus oculatus occupies less than 50% of the surface of Guanajuato and is also categorized as least concern in the Red List of Threatened Species (IUCN, 2016). Sciurus oculatus is found only within Mexico, and is under special protection in national legislation NOM-059-SEMARNAT-2010, update of 2019 (DOF, 2019). Both species face continuing decline in the extent and quality of habitat due to land use change (Conafor, 2020; IUCN, 2016, 2017), which is exacerbated by repeated burning episodes that decrease the area occupied by each species by 13-18%. According to Zamudio (2012), most of the plant communities in the state of Guanajuato have significantly changed in their structure, floristic composition and physiognomy. Consequently, their distribution areas have been gradually reduced. Currently, 63% of the territory has been transformed into agricultural areas, human settlements, and areas devoid of vegetation (Roth et al., 2016).
The recurrence of wildfires, mainly in the southern part of Guanajuato, represents an important threat to biodiversity conservation within PNA, which are surrounded by a complex matrix of rainfed and irrigated agricultural land uses. This result was also found by Farfán et al. (2020, 2021), where both the probabilities of anthropogenic ignition and climate under the ENSO climate conditions lead to high wildfire risk in this region of the state. These PNA urgently need fire management plans that can integrate fire prevention actions at the local level in the context of global warming. On a global scale, wildfires have been responsible for up to 27% of the loss of tree cover between 2001 and 2021 (Tyukavina et al., 2022). In Mexico alone, in 2021, 408.75 km2 of forest were lost due to fire (Tyukavina et al., 2022). The effects of wildfires on biodiversity patterns could be understimated if these shortfalls are underestimating the biodiversity. Therefore, actions at the international level are also urgent in order to prevent damage to unknown biogeographic patterns.
This is the first study for the state of Guanajuato and for Mexico that addresses the effect of wildfire on the potential distribution of 2 important taxonomic groups: amphibians and mammals. The evaluation of the Linnean and Wallacean shortfalls for any taxonomic group is essential before the identification of geographic patterns involved as criteria for conservation planning, even in terrestrial vertebrates which are assumed to be adequately sampled. The Wallacean shortfall could lead to underestimations of the effects of perturbations such as wildfires. This is particularly true of species that are already vulnerable due to anthropogenic factors such as land cover change, illegal trafficking, etc., as well as intrinsic factors like the size of their natural distributional areas, because it is unknown whether undersampling could represent geographically rare species. Improving the biogeographical knowledge of the patterns of amphibians and mammals could provide better tools to stakeholders in order to generate fire management plans to prevent the negative impact of the wildfire within protected areas around the world.
Acknowledgements
We thank Julián A. Velasco and Luis J. Aguirre for their help with the parametrization of Maxent, and Miguel Murgía for assistance with the quantification of the Qs estimator. We also thank to the anonymous reviewers and the Associate Editor for their careful reading of this manuscript.
References
Acevedo, P., Melo-Ferreira, J., Real, R., & Alves, P. C. (2014). Evidence for niche similarities in the allopatric sister species Lepus castroviejoi and Lepus corsicanus. Journal
of Biogeography, 41, 977–986. https://doi.org/10.1111/jbi.12
270
Aiello-Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B., & Anderson, R. P. (2015). spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography, 38,541–545. https://doi.org/10.1111/ecog.01132
Barlow, J., Berenguer, E., Carmenta, R. & França, F. (2020). Clarifying Amazonia’s burning crisis. Global Change Biology, 26, 319–321. https://doi.org/10.1111/gcb.14872op
Bivand, R., Keitt, T., Rowlingson, B., Pebesma, E., Sumner, M., Hijmans, R. et al. (2015). Package ‘rgdal’. Bindings for the Geospatial Data Abstraction Library. Retrieved on December, 2020 from: http://rgdal.r-forge.r-project.org
Chisholm, R. A., Wijedasa, L. S., & Swinfield, T. (2016). The need for long-term remedies for Indonesia’s forest fires. Conservation Biology, 30, 5–6. https://doi.org/10.1111/cobi.
12662
Chuvieco, E., Giglio, L. & Justice, C. (2008). Global characterization of fire activity: toward defining fire regimes from Earth observation data. Global Change Biology, 14, 1488–1502. https://doi.org/10.1111/j.1365-2486.2008.01585.x
Clivillé, S., Montori, A., Llorente, G. A., Santos, X., & Carretero, M. A. (1997). El impacto de los incendios forestales sobre los anfibios. Quercus, 138,10–13.
Cobos, M. E., Peterson, A. T., Barve, N., & Osorio-Olvera, L. (2019). Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ, 7,e6281. https://doi.org/10.7717/peerj.6281
Colwell, R. K., & Coddington, J. A. (1994). Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions of the Royal Society B, 345, 101–118. https://doi.org/10.1098/rstb.1994.0091
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2012). La biodiversidad en Guanajuato: estudio de estado (Vol. II). Guanajuato, Mexico: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Conabio)/ Instituto de Ecología del Estado de Guanajuato (IEE).
Conafor (Comisión Nacional Forestal). (2020). Estimación de la tasa de deforestación bruta en México para el periodo 2001-2018 mediante el método muestreo. Documento técnico. Comisión Nacional Forestal. Jalisco, México. Retrieved in 2022 from: http://www.conafor.gob.mx:8080/documentos/docs/1/7768Documento%20tecnico%202020%20Deforestacion%20Bruta%20Final.pdf
DOF (Diario Oficial de la Federación). (2010). NORMA
Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para
su inclusión, exclusión o cambio-Lista de especies en
riesgo. Mexico City, Mexico. Retrieved on March 3rd, 2021 from: https://dof.gob.mx/nota_detalle_popup.php?
codigo=5173091
DOF (Diario Oficial de la Federación). (2019). Modificación del Anexo Normativo III, Lista de especies en riesgo de la Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo, publicada el 30 de diciembre de 2010. Secretaría de Gobernación (SEGOB). Mexico City, Mexico. Retrieved on March 03rd, 2021 from: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019#gsc.tab=0
Diniz-Filho, J. F, Jardim, L., Guedes, J. J., Meyer, L., Stropp, J., Frateles, L. E. et al. (2023). Macroecological links between the Linnean, Wallacean, and Darwinian shortfalls. Frontiers of Biogeography, 15, e59566. http://dx.doi.org/10.21425/F5FBG59566
Diniz-Filho, J. A. F., Loyola, R. D., Raia, P., Mooers, A. O., & Bini, L. M. (2013) Darwinian shortfalls in biodiversity conservation. Trends in Ecology and Evolution, 28, 689–695. https://doi.org/10.1016/j.tree.2013.09.003
Escalante, T., Noguera-Urbano, E. A., & Corona, W. (2018). Track analysis of the Nearctic region: Identifying complex areas with mammals. Journal of Zoological Systematics and Evolutionary Research, 56, 466–477. https://doi.org/10.1111/jzs.12211
Escalante, T., Varela-Anaya, A. M., Noguera-Urbano, E. A., Elguea-Manrique, L. M., Ochoa-Ochoa, L. M., Gutiérrez-Velázquez, A. L. et al. (2020). Evaluation of five taxa as surrogates for conservation prioritization in the Trans-
mexican Volcanic Belt, México. Journal for Nature Conservation, 54, e125800. https://doi.org/10.1016/j.jnc.20
20.125800
Farfán, M., Pérez-Salicrup, D. R., Flamenco-Sandoval, A., Nicasio-Arzeta, S., Mas, J. F., & Ramírez-Ramírez, I. (2018). Modeling anthropic factors as drivers of wildfire occurrence at the Monarch Butterfly Biosphere. Madera y Bosques, 24, 1–15. https://doi.org/10.21829/myb.2018.2431591
Farfán, M., Flamenco-Sandoval, A., Rodríguez-Padilla, C. R., Rodrigues-de Sousa Santos, L., González-Gutiérrez, I., & Gao, Y. (2020). Cartografía de la probabilidad de ocurrencia a incendios forestales para el estado de Guanajuato: Una aproximación antrópica de sus fuentes de ignición. Acta Universitaria, 30, 1–15. https://doi.org/10.15174/au.2020.2953
Farfán, M., Domínguez, C., Espinoza, A., Jaramillo, A., Alcántara, C., Maldonado, V. et al. (2021). Forest fire probability under ENSO conditions in a semi-arid region: a case study in Guanajuato. Environmental Monitoring and Assessment, 193, 1–14. https://doi.org/10.1007/s10661-021-09494-0
Farrús, M., Anguita, J., Hernando, J., & Cerdà, R. (2007). Fusión de sistemas de reconocimiento basados en características de alto y bajo nivel. In III Congreso da Sociedade Española de Acústica Forense: actas do Congreso; 2005 oct 27-28; Santiago de Compostela, España. Santiago de Compostela: Dirección Xeral de Creación e Difusión Cultural.
Ferreira, B. M., Soares-Filho, B. S., & Quintão, F. M. (2019). The Dinamica EGO virtual machine. Science of Computer Programming, 173,3–20. https://doi.org/10.1016/j.scico.20
18.02.002
Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37,4302–4315. https://doi.org/10.1002/joc.5086
Flores-Villela, O., & Ochoa-Ochoa, L. M. (2020). Compilación de bases de datos de la herpetofauna mexicana. Museo de Zoología “Alfonso L. Herrera”, Facultad de Ciencias, Universidad Nacional Autónoma de México. Ciudad de México.
GBIF.org. (2020a). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
bbt2tq
GBIF.org. (2020b). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
j9evdt
GBIF.org. (2020c). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
z8nbtw
GBIF.org. (2020d). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
x4qxcx
GBIF.org. (2020e). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
apt9wm
GBIF.org. (2020f). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
bugsgy
GBIF.org. (2020g). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
x33mhm
GBIF.org. (2020h). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
esbshy
GBIF.org. (2020i). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
ju93kd
GBIF.org. (2020j). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
jrv9uw
GBIF.org. (2020k). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
gmjecq
GBIF.org. (2020l). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
q7kvhy
GBIF.org. (2020m). GBIF Occurrence Download Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
jjrncx
GBIF.org. (2020n). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
7hypb5
GBIF.org. (2020o). GBIF Occurrence Download Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
vncwkt
GBIF.org. (2020p). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
sbrrgp
GBIF.org. (2020q). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
t6y7vb
GBIF.org. (2020r). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
ksdpx3
GBIF.org. (2020s). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
uy2d7k
GBIF.org. (2020t). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
py7yby
GBIF.org. (2020u). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
8c8umf
GBIF.org. (2020v). GBIF Occurrence Download. Retrieved on November 11th, 2020 from: https://doi.org/10.15468/dl.
t92jw8
GBIF.org. (2020w). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
wumbut
GBIF.org. (2020x) GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.8tc8xr
GBIF.org. (2020y). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
tb25b4
GBIF.org. (2020z). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
paayuf
GBIF.org. (2020aa). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
8fzeuv
GBIF.org. (2020ab). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
vfa2qq
GBIF.org. (2020ac). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
ug5cus
GBIF.org. (2020ad). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
gpsjwg
GBIF.org. (2020ae). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
4ufzz3
GBIF.org. (2020af). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
dwvs69
GBIF.org. (2020ag). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.
upjycdg
GBIF.org. (2022ah). GBIF Occurrence Download. Retrieved on December 7th, 2020 from: https://doi.org/10.15468/dl.6tt8z6
Geary, W. L., Tulloch, A. I. T., Ritchie, E. G., Doherty, T. S., Nimmo, D. G., Maxwell, M. A. et al. (2023). Identifying historical and future global change drivers that place species recovery at risk. Global Change Biology, 29, 2953–2967. https://doi.org/10.1111/gcb.16661
González, T. M., González-Trujillo, J. D., Muñoz, A., & Armenteras, D. (2021). Differential effects of fire on the occupancy of small mammals in neotropical savanna-gallery forests. Perspectives in Ecology and Conservation, 19, 179–188. https://doi.org/10.1016/j.pecon.2021.03.005
Gotelli, N. J., & Colwell, R. K. (2011). Estimating species richness. In A. E. Magurran, & B. J. McGill (Eds.), Biological diversity: frontiers in measurement and assessment (pp. 39–54). Oxfordshire, England: Oxford University Press.
He, T., Lamont, B. B., & Pausas, J. G. (2019). Fire as a key driver of Earth’s biodiversity. Biological Reviews, 94, 1983–2010. https://doi.org/10.1111/brv.12544
Hortal, J., De Bello, F., Diniz-Filho, J. A. F., Lewinsohn, T. M., Lobo, J. M., & Ladle, R. J. (2015). Seven Shortfalls that Beset Large-Scale Knowledge of Biodiversity. Annual Review of Ecology, Evolution, and Systematics, 46, 523–549. https://doi.org/10.1146/annurev-ecolsys-112414-054400
Hu, F. S., Higuera, P. E., Duffy, P., Chipman, M. L., Rocha, A. V., Young, A. M., & Dietze, M. C. (2015). Arctic tundra fires: natural variability and responses to climate change. Frontiers in Ecology and the Environment, 13, 369–377. https://doi.org/10.1890/150063
IUCN (International Union for Conservation of Nature). 2001. IUCN Red List categories and criteria: Version 3.1. Prepared by IUCN Species Survival Commission. World Conservation Union, Gland, Switzerland and Cambridge, United Kingdom.
IUCN (International Union for Conservation of Nature). (2012). IUCN Red List Categories and Criteria: Version 3.1. (2nd ed.). IUCN, Gland, Switzerland.
IUCN (International Union for Conservation of Nature). (2016). Sciurus oculatus. The IUCN Red List of Threatened Species. Retrieved in 2022 from: e.T20017A22246721. https://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T20017A22246721.en
IUCN (International Union for Conservation of Nature). (2017). Sorex saussurei. The IUCN Red List of Threatened Species. Retrieved in 2022 from: e.T41416A22317311. https://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T41416A22317311.en
Jain, A., Nandakumar, K., Ross, A. (2005). Score normalization in multimodal biometric systems. Pattern Recognition, 38, 2270–2285. https://doi.org/10.1016/j.patcog.2005.01.012
Joppa, L. N., Roberts, D. L., Myers, N., & Pimm, S. L. (2011). Biodiversity hotspots house most undiscovered plant species. Proceedings of the National Academy of Sciences, 108, 13171–13176. https://doi.org/10.1073/pnas.1109389108
Kass, J. M., Vilela, B., Aiello-Lammens, M. E., Muscarella, R., Merow, C., & Anderson, R. P. (2017). Wallace: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods in Ecology and Evolution, 9, 1151–1156. https://doi.org/10.1111/2041-210X.12945
Kelly, L. T., Giljohann, K. M., Duane, A., Aquilué, N., Archibald, S., Batllori, E. et al. (2020). Fire and biodiversity in the Anthropocene. Science, 370, 1–10. https://doi.org/10.1126/science.abb0355
Kindt, R., & Coe, R. (2005). Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. Nairobi, Kenya: World Agroforestry Centre (ICRAF).
Leyte-Manrique, A., Mata-Silva, V., Baez-Montes, O., Fucsko, L. A., DeSantis, D. L., Garcia-Padilla, E. et al. (2022). The herpetofauna of Guanajuato, Mexico: composition, distribution, and conservation status. Amphibian & Reptile Conservation, 16, 133–180.
Lomolino, M. V. (2004). Conservation biogeography. In M. V. Lomolino, & L. R. Heaney (Eds.), Frontiers of biogeography: new directions in the geography of nature (pp. 293–296). Sunderland, Massachusetts: Sinauer.
Luedtke, J. A., Chanson, J., Neam, K., Hobin, L., Maciel, A. O., Catenazzi, A. et al. (2023). Ongoing declines for the world’s amphibians in the face of emerging threats. Nature, 622, 308–314. https://doi.org/10.1038/s41586-023-06578-4
Mandeville, P. B. (2008). Tips bioestadísticos: tema 18 ¿Por qué se deben centrar las covariables en regresión lineal? Ciencia UANL, 11, 300–305.
Martínez-Torres, H., Cantú-Férnandez, M., Ramírez, M. I., & Pérez-Salicrup, D. R. (2015). Fires and fire management in the Monarch Butterfly Biosphere Reserve. In K. S. Oberhauser, K. R. Nail, & S. Altizer (Eds.), Monarchs in a changing world: biology and conservation of an iconic butterfly (pp. 179–189). Ithaca, New York: Cornell University Press.
Montgomery, D., & Peck, E. A. (1992). Introduction to linear regression analysis. Second Edition. Hoboken, Nueva Jersey: John Wiley & Sons Ltd.
Morrone, J. J., Escalante, T., & Rodríguez-Tapia, G. (2017). Mexican biogeographic provinces: map and shapefiles. Zootaxa, 4277, 277–279. https://doi.org/10.11646/zootaxa.
4277.2.8
Murguía-Romero, M., & Villaseñor, J. L. (2000). Estimating the quality of the records used in quantitative biogeography with presence-absence matrices. Annales Botanici Fennici, 37, 289–296.
Naimi, B., Hamm, N. A. S., Groen, T. A., Skidmore, A. K., & Toxopeus, A. G. (2014). Where is positional uncertainty a problem for species distribution modelling? Ecography, 37, 191–203. https://doi.org/10.1111/j.1600-0587.2013.00205.x
Nasa Earth Data Cloud. (2020). Datos de incendios activos. NASA Official: Cerese Alber Retrieved on November 3rd, 2021 from: https://earthdata.nasa.gov/earth-observation-data/near-real-
time/firms/activefiredata
Oliveira, U., Paglia, A. P., Brescovit, A. D., de Carvalho, C. J. B., Silva, D. P., Rezende, D. T. et al. (2016). The strong influence of collection bias on biodiversity knowledge shortfalls of Brazilian terrestrial biodiversity. Diversity and Distributions, 22,1232–1244. https://doi.org/10.1111/ddi.12489
Osorio-Olvera, L., Lira-Noriega, A., Soberón, J., Peterson, A. T., Falconi, M., Contreras-Díaz, R. G. et al. (2020). NTBOX: an R package with graphical user interface for modelling and evaluating multidimensional ecological niches. Methods in Ecology and Evolution, 11, 1199-1206. https://doi.org/10.1111/2041-210X.13452
Pearson, R. G., Raxworthy, C. J., Nakamura, M., & Townsend, A. (2007). Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography, 34, 102–117. https://doi.org/10.1111/j.1365-2699.2006.01594.x
Peterson, A. T., Papeş, M., & Soberón, J. (2008). Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecological Modelling, 213,63–72. https://doi.org/10.1016/j.ecolmodel.2007.11.008
Pilliod, D. S., Bury, R. B., Hyde, E. J., Pearl, C. A., & Corn, P. S. (2003). Fire and amphibians in North America. Forest Ecology and Management, 178, 163–181. https://doi.org/10.1016/S0378-1127(03)00060-4
Pyne, S. J. (2021). The Pyrocene: how we created an Age of Fire, and what happens next. Oakland, California: University of California Press.
QGIS Development Team. (2020). QGIS Geographic Information System. Open Source Geospatial Foundation Project. Beaverton, Oregon. Retrieved on March 2nd, 2021 from: https://qgis.org
Riddle, B. R., Ladle, R. J., Lourie, S. A., & Whittaker, R. J. (2011). Basic Biogeography: estimating biodiversity and mapping nature. In R. J. Ladle, & R. J. Whittaker (Eds.), Conservation Biogeography (pp. 45–92). Hoboken, New Jersey: Wiley-Blackwell. https://doi.org/10.1002/9781444390001.ch4
Rocchini, D., Hortal, J., Lengyel, S., Lobo, J. M., Jiménez-Valverde, A., Ricotta, C. et al. (2011). Accounting for uncertainty when mapping species distributions: the need for maps of ignorance. Progress in Physical Geography: Earth and Environment, 35, 211–226. https://doi.org/10.
1177/0309133311399491
Rodrigues, J. F. M., Villalobos, F., Iverson, J. B., & Diniz-Filho, J. A. F. (2019). Climatic niche evolution in turtles is characterized by phylogenetic conservatism for both aquatic and terrestrial species. Journal of Evolutionary Biology, 32,66–75. https://doi.org/10.1111/jeb.13395
Roth, D., Moreno-Sánchez, R., Torres-Rojo, J. M., & Moreno-Sánchez, F. (2016). Estimation of human induced disturbance of the environment associated with 2002, 2008 and 2013 land use/cover patterns in Mexico. Applied Geography, 66, 22–34. https://doi.org/10.1016/j.apgeog.2015.11.009
RStudio Team. (2020). RStudio: integrated development for R. RStudio, PBC, Boston, Massachusetts. Retrieved in 2020 from: http://www.rstudio.com/
Salazar, D. N., Farfán-Gutiérrez, M., & Arellano-Reyes, M. A. (2019). Cartografía de la severidad de los incendios forestales (2017, 2018, 2019) en el estado de Guanajuato empleando imágenes Sentinel-2. Jóvenes en la Ciencia, 5, 1-6.
Sánchez O., Charre-Medellín, J. F., Téllez-Girón, G., Báez-Montes, Ó., & Magaña-Cota, G. (2016). Mamíferos silvestres de Guanajuato: actualización taxonómica y diagnóstico de conservación. In M. Briones-Salas, Y. Hortelano-Moncada, G. Magaña-Cota, G. Sánchez-Rojas, & J. E. Sosa-Escalante, (Eds.), Riqueza y conservación de los mamíferos en México a nivel estatal (pp. 243–280). Mexico City: Instituto de Biología, UNAM/ Asociación Mexicana de Mastozoología/ Universidad de Guanajuato.
Santos-Barrera, G., & Flores-Villela, O. (2004). Lithobates megapoda. IUCN Red List of Threatened Species. Versión 2013.2. Cambridge, United Kingdom. Retrieved on April 1st, 2021 from: www.iucnredlist.org
SMAOT (Secretaría de Medio Ambiente y Ordenamiento Territorial). (2022). Áreas Naturales Protegidas. Gobierno del Estado de Guanajuato, Guanajuato. Retrieved on October 26th, 2022 from: https://smaot.guanajuato.gob.mx/sitio/areas-naturales-protegidas
Soberón, J., & Peterson, A. T. (2005). Interpretation of Models of Fundamental Ecological Niches and Species’ Distributional Areas. Biodiversity Informatics, 2, 1–10. https://doi.org/10.17161/bi.v2i0.4
Shlisky, A., Waugh, J., González, P., González, M., Manta, M., Santoso, H. et al. (2007). Fire, ecosystems and people: threats and strategies for global biodiversity conservation. Global Fire Initiative Technical Report 2007-2; Arlington, VA: The Nature Conservancy.
Tessarolo, G., Ladle, R. J., Lobo, J. M., Rangel, T. F. & Hortal, J. (2021), Using maps of biogeographical ignorance to reveal the uncertainty in distributional data hidden in species distribution models. Ecography, 44, 1743–1755. https://doi.org/10.1111/ecog.05793
Troudet, J., Grandcolas, P., Blin, A., Vignes-Lebbe, R., & Legendre, F. (2017). Taxonomic bias in biodiversity data and societal preferences. Scientific Reports, 7,1–14. https://doi.org/10.1038/s41598-017-09084-6
Tyukavina, A., Potapov, P., Hansen, M. C., Pickens, A. H., Stehman, S. V., Turubanova, S. et al. (2022). Global trends
of forest loss due to fire from 2001 to 2019. Frontiers
in Remote Sensing, 3,1–20. https://doi.org/10.3389/frsen.
2022.825190
United States Geological Survey (USGS). (2021). Archivo EROS de USGS-Elevación digital- Elevación global de 30 segundos de arco (GTOPO30). Retrieved on June 18th, 2021 from: https://www.usgs.gov/centers/eros/science/usgs-eros-archive-digital-elevation-global-30-arc-second-elevation-gtopo30
Vergara-Asenjo, G., Alfaro, F. M., & Pizarro-Araya, J. (2023) Linnean and Wallacean shortfalls in the knowledge of arthropod species in Chile: Challenges and implications for regional conservation. Biological Conservation, 281, 110027. https://doi.org/10.1016/j.biocon.2023.110027
Warren, D. L., & Seifert, S. N. (2011). Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological Applications, 21,335–342. https://doi.org/10.1890/10-1171.1
Zamudio, S. (2012). Diversidad de ecosistemas del estado de Guanajuato. In Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Conabio)/ Instituto de Ecología del estado de Guanajuato (IEE) (Eds.), La biodiversidad de Guanajuato: estudio de estado. II (pp. 19–55). Mexico City: Conabio/ IEE.
Monitoreo poblacional y estado de conservación de la ranita del Pehuenche (Alsodes pehuenche) en el valle Pehuenche, Mendoza, Argentina
Gabriela Diaz a, b, *, Vanesa Pellegrini-Piccini a, Liliana Moreno d, Martín Palma c, e, Vanesa Bentancourt c y Valeria Corbalán f
a Universidad Nacional de Cuyo-Sede Malargüe, Campus Educativo Municipal, Facultad de Ciencias Exactas y Naturales, Rosario Vera Peñaloza y Beltrán, 5613 Malargüe, Mendoza, Argentina
b Universidad Nacional de Cuyo, Instituto de Ingeniería y Ciencias Aplicadas a la Industria-CONICET, Facultad de Ciencias Aplicadas a la Industria, Bernardo de Yrigoyen Núm. 375, 5600 San Rafael, Mendoza, Argentina
c Instituto de Educación Física Núm. 9-016 “Jorge E. Coll” Dirección General de Escuelas-Sede Malargüe, Tecnicatura en Conservación de la Naturaleza, Campus Educativo Municipal, Rosario Vera Peñaloza y Beltrán, 5613 Malargüe, Mendoza, Argentina
d Universidad Nacional de San Luis, Facultad de Química Bioquímica y Farmacia, Ejército de los Andes Núm. 950, 5700 San Luis, Argentina
e Ministerio de Ambiente y Desarrollo Sustentable de la Provincia de Mendoza, Dirección de Recursos Naturales Renovables, Delegación Malargüe, San Martín Norte Núm. 352, 5613 Malargüe, Mendoza, Argentina
f Instituto Argentino de Investigaciones de Zonas Áridas (CCT Mendoza-CONICET), Av. Ruiz Leal s/n, Parque Gral. San Martín, 5500 Mendoza, Argentina
*Autor para correspondencia: gdiaz@infoar.net (G. Diaz)
Recibido: 02 octubre 2023; aceptado: 15 agosto 2024
Resumen
La ranita del Pehuenche, Alsodes pehuenche, es endémica de los Andes centrales de Argentina y Chile, ha sido categorizada en peligro crítico por la UICN y entre sus amenazas se encuentran la ruta internacional que atraviesa los arroyos que habita, la presencia del hongo quitridio, los salmónidos exóticos invasores, el ganado y el cambio climático. El objetivo de este trabajo fue evaluar el estado actual de conservación de A. pehuenche en el valle Pehuenche para conocer tendencias poblacionales, el impacto de las amenazas y futuras acciones de manejo. Se realizaron 14 salidas de campo durante 3 temporadas (2021-2023) y se muestrearon 12 arroyos usando la técnica de encuentro visual nocturno. Se delimitaron y nombraron 7 subpoblaciones: Nacientes, del Límite, Pichintur, Rial Rojas, Nueva, Campanaria y Cajón Largo. Los resultados muestran conteos de adultos (5.82 en 200 m2 y 13.64 por hora) y de larvas (6.24 en 200 m2 y 17.76 por hora). Éstos no variaron significativamente entre temporadas, pero fueron mayores en enero y febrero. Con base en la conectividad y las amenazas, los índices del estado de conservación permiten priorizar las subpoblaciones como unidades de conservación, de las cuales la del Límite requiere esfuerzos más urgentes.
Palabras clave: Especie amenazada; Encuentro visual; Conectividad; Subpoblaciones; Priorización de conservación
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Population monitoring and conservation status of the Pehuenche frog (Alsodes pehuenche) in the valle Pehuenche, Mendoza, Argentina
Abstract
The Pehuenche spiny-chest frog, Alsodes pehuenche, is endemic to the Central Andes of Argentina and Chile. It has been categorized as critically endangered by the IUCN and its threats include the international road that crosses the streams inhabited by the species, the presence of the chytrid fungus, invasive exotic salmonids, livestock, and climate change. The objective of this work was to evaluate the current conservation status of A. pehuenche in the Pehuenche Valley as a basis for understanding population trends, the impact of threats, and future management actions. Fourteen field trips were conducted during 3 seasons (2021-2023) and 12 streams were sampled using the nocturnal visual encounter technique. Seven subpopulations were delimited and named: Nacientes, del Límite, Pichintur, Rial Rojas, Nueva, Campanaria, and Cajón Largo. The results show counts of adults (5.82 in 200 m2 and 13.64 per hour) and larvae (6.24 in 200 m2 and 17.76 per hour). These did not vary significantly between seasons but were higher in January and February. According to connectivity and threats, the conservation status indices allow us to prioritize the subpopulations as conservation units, with del Límite being the one that requires the most urgent efforts.
Keywords: Threatened species; Visual encounter; Connectivity; Subpopulations; Conservation prioritization
Introducción
Los anfibios son el grupo de vertebrados más amenazados de nuestro planeta, varias son las causas responsables de la disminución de sus poblaciones (Grant et al., 2020; Green et al., 2020; Luedtke et al., 2023). Más de 45% de la diversidad de anfibios del mundo se distribuyen en el Neotrópico (Kacoliris et al., 2022). Alrededor de 25% de las especies de Argentina son endémicas (Vaira et al., 2017), 37% de ellas en Argentina y Chile se encuentran en disminución, mientras que del 22% de las especies no se conoce su tendencia poblacional (Kacoliris et al., 2022). Las amenazas más importantes con las que se asocian la disminución poblacional o extinciones locales son los depredadores invasores, enfermedades emergentes y la ganadería (Kacoliris et al., 2022; Velasco et al., 2016).
La ranita del Pehuenche, Alsodes pehuenche, fue descrita por Cei (1976). Luego de estudios citogenéticos realizados entre 1983 y 2003 (Cuevas y Formas, 2003), se renueva el interés sobre la especie en Argentina debido a las obras viales sobre la ruta internacional ARG145 – CH115, cuya pavimentación desvió el curso de 5 afluentes del arroyo Pehuenche con presencia de la especie (Corbalán et al., 2010). La ranita del Pehuenche es una especie endémica y su distribución está restringida a los Andes centrales de Argentina y Chile (Corbalán et al., 2010, 2023; Correa et al., 2013, 2018, 2020). Habita arroyos de montaña en ecosistemas de vegas o mallines entre 2,150 y 2,825 m snm (Corbalán et al., 2023). Los arroyos poseen lechos pedregosos y una fina capa de sedimentos. La especie presenta dimorfismo sexual y como otras especies de Alsodes, tiene larvas de desarrollo prolongado con juveniles y adultos de hábitos acuáticos (Cei, 1976, 1980; Herrera y Velázquez, 2016a; Úbeda, 2021). Las larvas son de gran tamaño y pasan al menos 4 años en los cuerpos de agua permanentes hasta completar el ciclo larval (Corbalán et al., 2014). Se ha reportado la puesta de huevos ocultos bajo rocas o en oquedades en las márgenes de los arroyos (Corbalán et al., 2014; Piñeiro et al., 2020). Estas cavidades son utilizadas también como refugio por los adultos (Cei, 1980; Correa et al., 2013; Herrera y Velázquez, 2016b).
Los datos de conteos disponibles corresponden a muestreos de tramos cortos de arroyos en Argentina y Chile (Corbalán et al., 2010, 2023; Correa et al., 2013). La coexistencia de individuos en una cavidad durante el día sugiere densidades elevadas en una categoría de microhábitat (Correa et al., 2013). Han ocurrido eventos de mortalidad y se han reportado nuevas poblaciones (Corbalán et al., 2023; Correa et al., 2018), lo cual hace necesaria una evaluación actualizada de su estatus de conservación y tendencia poblacional.
Las evaluaciones del estado de conservación de la ranita del Pehuenche la han colocado en la categoría más alta de amenaza: “en peligro” por la Asociación Herpetológica Argentina (Vaira et al., 2012), cuarta en orden de prioridad entre los 58 anfibios evaluados de Chile (Vidal et al., 2024) y “en peligro crítico” por la Unión Internacional para la Conservación de la Naturaleza (IUCN, 2019), según los criterios B1ab basados en su extensión de presencia estimada y su disminución continua estimada, provocada principalmente por la pavimentación de la ruta. El área de ocupación de la especie (AOO sensu UICN) estimada actualmente es de 4.84 km2 y su extensión de presencia (EOO sensu UICN) 497.9 km2 (Corbalán et al., 2023). En cuanto a las amenazas consideradas en la categorización de IUCN (2019), se enumeran el desvío de los cursos de agua por la construcción de la ruta, el impacto del ganado, el cambio climático, la presencia del hongo quitridio (Batrachochytrium dendrobatidis) y la depredación por salmónidos exóticos invasores tales como la trucha arcoíris (Oncorhynchus mykiss)y trucha marrón (Salmo trutta) (Corbalán et al., 2023; Ghirardi et al., 2014; Zarco et al., 2020).
Si bien los avances en el conocimiento de la distribución de A. pehuenche han sido importantes en los últimos años, aún se desconocen aspectos básicos de la ecología, sistemática, reproducción, comportamiento y estrategias ecofisiológicas de esta especie en los humedales de altura. Por tratarse de una especie endémica y amenazada, la historia de vida y demografía son fundamentales para la evaluación del estatus de conservación (Luja et al., 2015). Los programas de seguimiento de poblaciones son necesarios para identificar y detectar disminuciones que amenacen la persistencia de poblaciones y deben llevarse a cabo en un marco de gestión adaptativa que permita realizar monitoreos que maximicen la detección y minimicen el esfuerzo (Pollock, 2006, Yoccoz et al., 2001). A su vez, la definición de subpoblaciones es una herramienta útil que permite definir su estado actual y priorizar acciones de conservación (Velasco, 2018).
El objetivo de este trabajo fue evaluar el estado de conservación actual de A. pehuenche en el valle Pehuenche. Esta información es fundamental para estimar tendencias poblacionales a largo plazo, evaluar el impacto de las amenazas y el éxito de futuras acciones de manejo. En este trabajo se muestran los primeros datos del programa de monitoreo iniciado en 2021 en el valle Pehuenche. A partir del mismo, se definen y delimitan subpoblaciones como unidades de conservación sobre las que se deben priorizar las acciones.
Materiales y métodos
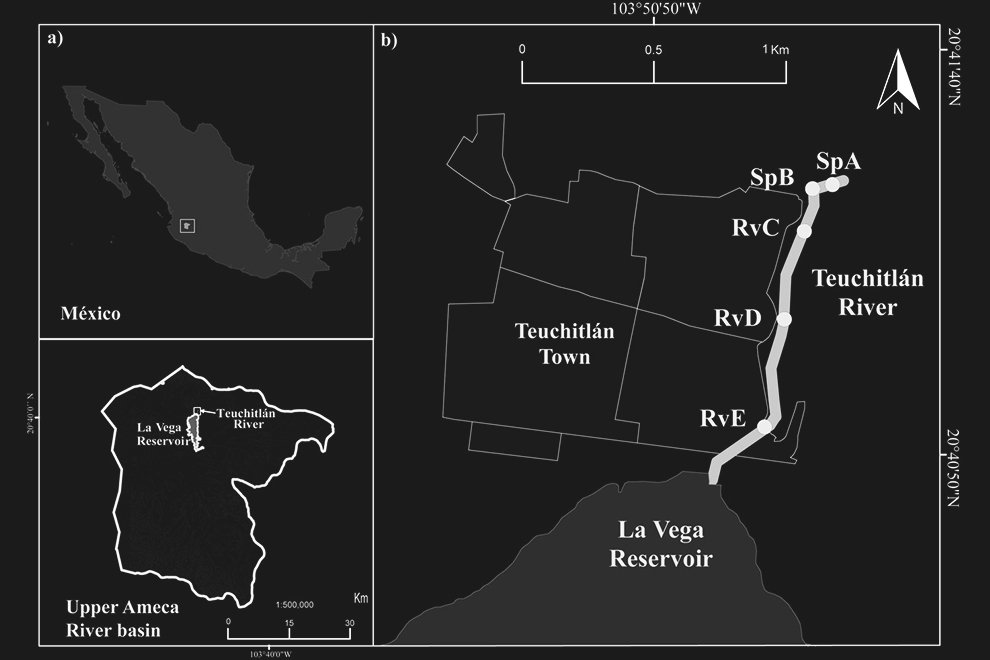
El área de estudio corresponde al valle Pehuenche, en el lado argentino de la zona limítrofe entre Argentina y Chile; forma parte de los Andes centrales, en el departamento de Malargüe, suroeste de la provincia de Mendoza. Las precipitaciones anuales son de 400 a 600 mm (Rivera et al., 2018) y están influidas por los vientos provenientes del Pacífico sur, creando un gradiente de precipitación y humedad de oeste a este con fuertes nevadas en invierno (Garreaud et al., 2009). Durante la primavera-verano, el deshielo alimenta los humedales, denominados localmente vegas o mallines, donde se asientan familias con su ganado en los puestos, denominados reales o riales. El área es considerada un corredor ecológico y cultural trashumante de gran importancia (Llano et al., 2021).
La cuenca del arroyo Chico en Argentina incluye las subcuencas donde se distribuye A. pehuenche: arroyos Pehuenche y Callao (Corbalán et al., 2023). El arroyo Chico es afluente del río Grande en el departamento de Malargüe, provincia de Mendoza. En Chile, el área de distribución de la especie se ubica en la cuenca del río Maule, con 2 subpoblaciones posiblemente aisaldas: laguna del Maule y Lo Aguirre (Correa et al., 2013, 2018).
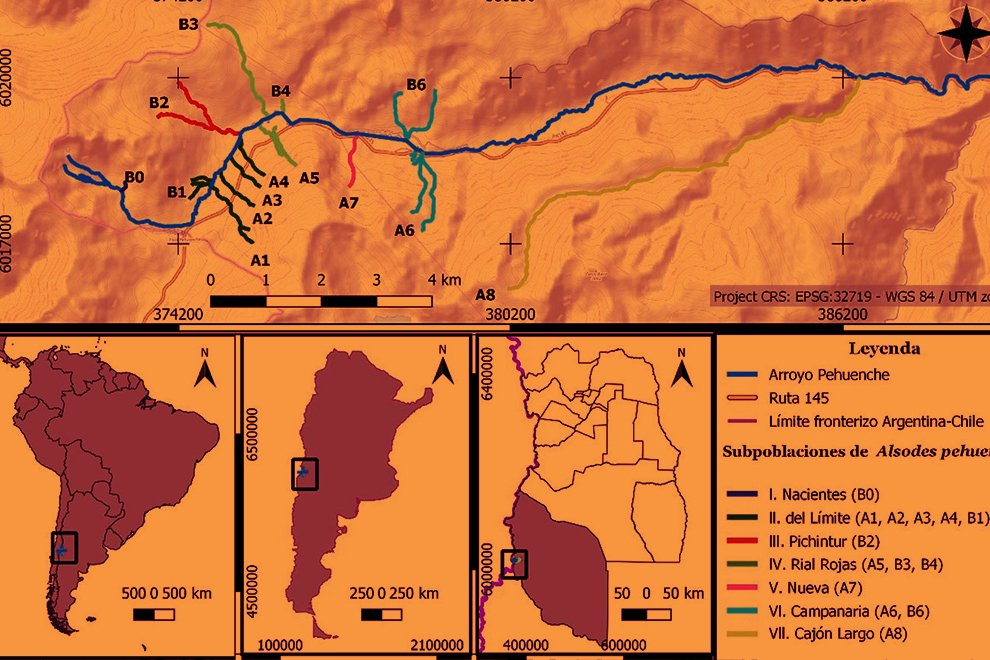
Durante 3 temporadas se muestrearon 14 arroyos tributarios del arroyo Pehuenche (fig. 1). Doce de los 14 arroyos fueron muestreados exhaustivamente y en los 2 restantes (B0 y A8), solo se constató la presencia de la especie. La especie fue observada en la desembocadura de los arroyos de primer orden en el arroyo Pehuenche.
Siguiendo a Corbalán et al. (2010) y Prado et al. (2019), los arroyos de primer orden que desembocan en el arroyo Pehuenche se distinguen, según su ubicación respecto a este último, en arroyos A que se ubican hacia el sur y son atravesados por la ruta Núm. 145, mientras que los arroyos B se ubican al norte del arroyo Pehuenche (fig. 1). A los arroyos con bifurcaciones y cursos de agua paralelos a menos de 100 m de distancia se los denominó con el mismo nombre. Durante el estudio, se tomaron datos de temperatura del agua con termómetro de mercurio, así como pH, oxígeno disuelto y conductividad con sonda multiparamétrica Lutron WA-2017SD, y de temperatura ambiente mediante la aplicación yr (Jensen et al., 2007). La conductividad fue baja, entre 0 y 181.56 μS/cm (n = 65), el valor medio del pH estuvo cercano a la neutralidad 6.41 ± 1.2, variando entre 3.29 y 8.25 (n = 77) y la concentración de oxígeno disuelto varió entre 1.3 y 15.8 mg/l (n = 16). La temperatura ambiente durante los muestreos osciló entre 2 y 21 ºC (n = 89), y la temperatura del agua entre 2 y 17.6 ºC (n = 75).
Se realizaron un total de 14 salidas de campo con una duración de 2 a 4 días/noches (tabla 1). Las 14 salidas se distribuyeron en 3 temporadas; cada una se inicia con el deshielo (octubre-noviembre-diciembre) y finaliza con la caída de las primeras nevadas (marzo-abril). En enero de 2021 se iniciaron muestreos preliminares diurnos y nocturnos consistentes en encuentros visuales sin manipulación de individuos. Los muestreos nocturnos iniciaron luego de la puesta del sol que coincide con el pico de actividad de los individuos adultos. Se optó por continuar con los muestreos nocturnos debido a que no se obtuvieron diferencias significativas en la cantidad de larvas entre el día y la noche (W = 191, p = 0.859).

Figura 1. Arroyos muestreados en la cuenca del valle Pehuenche, distribuidos en 7 subpoblaciones locales: I. Nacientes, II. del Límite, III. Pichintur, IV. Rial Rojas, V. Nueva, VI. Campanaria, VII. Cajón Largo. Los arroyos de cada subpoblación se indican con el mismo color.
Se contabilizaron adultos, juveniles y larvas usando la técnica de encuentro visual (Crump y Scott, 1994). Los transectos fueron de rumbo variable siguiendo el curso del arroyo y el ancho fue de 1 m a cada lado del arroyo. Cada transecto se realizó con 2 a 5 observadores. Siguiendo las recomendaciones de Pereyra et al. (2021), al menos un observador tenía experiencia previa y las salidas se programaron en días con condiciones meteorológicas similares: días sin lluvias, mayormente despejados y con vientos leves a moderados.
Para los estadios maduros se registró sexo (macho, hembra e indeterminado). Se registró punto GPS y hora de inicio y de finalización del transecto. Se calcularon las abundancias relativas como individuos/área e individuos/tiempo. El área de los arroyos se consideró como el tramo muestreado en metros multiplicado por 2 m de ancho. Los individuos contabilizados estuvieron a no más de 1 m de distancia del curso del arroyo, cuando éste está claramente definido, y solo se observaron más dispersos en las zonas húmedas de vegas. Para el cálculo de individuos/tiempo se usó el tiempo en minutos de la duración del muestreo (Pereyra et al., 2021).
Adicionalmente, se realizaron muestreos en transectos de 800 m de longitud en los márgenes de la calzada: 10 en la temporada estival de 2021 y 5 en las temporadas siguientes. Este registro se realizó ya que en enero de 2018 se observaron individuos deshidratados o muertos, adyacentes a un cordón o bordillo de 20 cm de altura y 15 cm de ancho.
Se definieron subpoblaciones, siguiendo a Velasco (2018), formadas por un grupo de arroyos que contienen individuos de A. pehuenche, con hábitat adecuado (sensu Corbalán et al., 2010; Correa et al., 2013) y cuya distancia entre ellos no fue mayor a 500 m lineales. De este modo, los 14 arroyos iniciales fueron agrupados en 7 subpoblaciones (fig. 1). Los nombres de las subpoblaciones se relacionan con referencias a las características del paisaje donde se encuentran, excepto la subpoblación denominada Nueva. Se calculó el índice de conectividad (IC) entre subpoblaciones de acuerdo con la fórmula de Lin (2009), modificada por Velasco (2018): IC = A1*(1/D1*T1) + A2*(1/D2*T2), donde Ai es el área (= tamaño poblacional) de la población local vecina hacia el lado i, Di es la distancia a la población local vecina hacia el lado i y Ti la presencia de truchas en hábitat intermedios (presencia multiplica por 2, ausencia multiplica por 1). Para estimar el área correspondiente a cada subpoblación, se usaron las coordenadas GPS de los muestreos de campo y en el caso de las 2 subpoblaciones donde solo se constató presencia, se estimó tamaño del área en Google Earth Pro.
Tabla 1
Temporadas y subpoblaciones en las que se realizaron los muestreos de Alsodes pehuenche. Se indican la cantidad de muestreos realizados en la ruta y los nombres de los arroyos (tipos A, B; P: arroyo Pehuenche) muestreados por subpoblación.
| Temporada | Mes | Quincena primera (1) segunda (2) | Ruta | Subpoblaciones | ||||||
| I | II | III | IV | V | VI | VII | ||||
| 1 (2021) | enero | 1 | 2 | B0 | A1, A2 | |||||
| enero | 2 | 6 | A1, A2, A3, A4 | A5 | A7 | |||||
| febrero | 2 | 1 | A1, A2 | B2 | B3 | |||||
| abril | 2 | 1 | A1, A2 | A5 | A8 | |||||
| 2 (2021-2022) | noviembre | 2 | 1 | A1, B1 | B2 | A5 | A7 | |||
| febrero | 1 | A2 | B3 | |||||||
| marzo | 1 | A1 | B2 | |||||||
| marzo | 2 | B1 | B3 | |||||||
| 3 (2022-2023) | noviembre | 2 | 1 | A5 | ||||||
| diciembre* | 1 | 1 | A1, A3, B1 | B2, B2 | A5-B3 | A6 | A7 | |||
| enero | 1 | 1 | A2, P | A5, B3, P | A6, P | A7, P | ||||
| febrero | 2 | 1 | A1, P | A7, P | ||||||
| marzo | 2 | A3, P | B4, P | B6, P |
Las amenazas que se registraron durante los conteos se consideraron de manera cualitativa, en una escala de 0 a 3, de acuerdo con la intensidad de la amenaza (Velasco, 2018). En la tabla 2 se muestran las amenazas consideradas: 1) la presencia de salmónidos que fueron registrados por encuentro visual; 2) la sequía observada de arroyos que podría ser reversible según la época del monitoreo y cárcavas permanentes cuya profundidad va en aumento; 3) la presencia de ganado, principalmente vacuno y caprino, el primero es el que podría causar mayor impacto debido al mayor uso de las vegas; 4) mortalidad de individuos por causas indeterminadas; 5) la ruta, considerada como una barrera para la dispersión, ya sea porque las alcantarillas no fueron diseñadas como pasos de fauna o porque la altura del cordón o bordillo construido sobre la misma es muy alto para el libre tránsito de ranas juveniles y adultas. Y a pesar de que la ruta representa una barrera, la construcción posterior de rampas, con el objetivo de mitigar este impacto, se considera una acción concreta de conservación. Se registró, además, la presencia de depredadores no acuáticos potenciales y de otras especies de anfibios, pero ninguno de estos casos se considera como amenaza, aunque podrían representar interacciones interespecíficas negativas.
El estado de conservación (EC) relativo de cada subpoblación se obtuvo a partir de la sumatoria de los valores asignados al índice de conectividad y las amenazas (Velasco, 2018). Los valores fueron estandarizados (ECE = EC subpoblación – promedio de todos los EC/desviación estándar de todos los EC) y el valor resultante se multiplicó por -1. Así, los valores negativos de ECE quedaron relacionados con situaciones menos favorables. Se establecieron 2 categorías de prioridades de conservación: baja a media (valores positivos de ECE) y alta (valores negativos de ECE). Los valores altos son los que requieren medidas más urgentes de manejo (Velasco, 2018).
Tabla 2
Variables consideradas para evaluar estado de conservación de las subpoblaciones de Alsodes pehuenche en el valle Pehuenche. IC: Índice de conectividad. Valores 0 a 3 son los que se le asignan a cada variable.
| Variables | 0 | 1 | 2 | 3 |
| Área (m2) | 7,500-10,000 | 5,000-7,500 | 2,500-5,000 | 1,000-2,500 |
| IC1 | 7.51-10 | 5.1-7.5 | 2.51-5 | 0-2.5 |
| Amenazas | ||||
| Efecto especies exóticas: salmónidos | sin | presencia registrada | presencia registrada en más de un conteo | presencia registrada y presencia de anfibio/s con signo de depredación |
| Fragmentación por la ruta | Sin ruta | Sin cordón o bordillo | Con cordón o bordillo y con rampas | Con cordón o bordillo y sin rampas |
| Pérdida de hábitat por sequía/ cárcavas | Sin evidencias de sequía ni cárcavas | Algún arroyo parcialmente seco | Algún arroyo parcialmente seco y/o con presencia cárcavas | Algún arroyo completamente seco y/o presencia de individuos muertos por sequía |
| Efecto del ganado | Sin ganado | Con caprinos | Con vacunos | Con vacunos y cabras |
| Indeterminada | Sin registro de individuos muertos sin causa aparente | Registro de individuos con manchas en la piel | Registro de individuos con manchas y uno muerto sin causa aparente | Registro de más de un individuo muerto sin causa aparente |
1 = Índice de conectividad calculado de acuerdo con Lin (2009), modificado por Velasco (2018).
Se realizaron análisis de los conteos con R versión 4.1.3 y los análisis estadísticos consideraron 0.05 de nivel de significación de alfa. La normalidad de los datos se verificó con la prueba de Shapiro-Wilk. La cantidad de individuos (adultos, juveniles y larvas) por área y por hora no se distribuyó normalmente (W < 0.82934, p < 0.001), por lo que se usaron las pruebas estadísticas no paramétricas de Mann-Whitney-Wilcoxon y de Kruskal-Wallis para 2 grupos y más de 2 grupos, respectivamente. Se evaluó estadísticamente si hubo diferencias entre adultos y juveniles considerados en conjunto versus larvas. También se evaluaron diferencias en relación con variables temporales (temporadas, meses) y espaciales (entre arroyos A y B y entre subpoblaciones). Cuando se obtuvieron diferencias significativas con la prueba de Kruskal-Wallis, se usó el paquete conover.test versión 1.1.5 (2017), basado en Conover-Iman Test para comparaciones múltiples. En todos los casos, los resultados se expresan en mediana, el rango intercuartílico (IQR) como medida de dispersión, los valores mínimos y máximos (min-max) y el tamaño de la muestra (n).
Resultados
Los transectos muestreados alcanzaron un total de 51,292 m lineales recorridos en 181 horas con un esfuerzo de muestreo 12.35 horas/persona. De los 14 arroyos estudiados, todos tuvieron presencia de la especie y el área total fue de 0.04 km². Más de la mitad corresponde a los arroyos denominados A (56.2%).
Cuando se analizaron los conteos respecto de la cantidad de observadores, la correlación de Spearman resultó en valores de rho cercanos a 0, lo que sugiere que no hay correlación lineal (p > 0.05), tanto para individuos postmetamórficos (por área: rho = -0.0427, n = 99; por hora: rho = 0.0975, n = 99), como para larvas (por área: rho = -0.0297, n = 92; por hora rho = -0.0063,
n = 92).
A lo largo del periodo de muestreo se observaron 5.82 adultos y juveniles/área (IQR = 9.27, 0-45 min-max, n = 99) y 13.64 adultos y juveniles/hora (IQR = 30.29, 0-108 min-max, n = 99); 6.24 larvas/área (IQR = 31, 0-175 min-max, n = 92) y 17.76 larvas/hora (IQR = 65, 0-367 min-max, n = 92). La comparación entre estadios muestra menos postmetamórficos que larvas, tanto para los conteos de individuos por área (W = 12.524, p < 0.001) como por hora (W = 26.918, p < 0.001).
Tabla 3
Prioridades de conservación para las subpoblaciones de Alsodes pehuenche en el valle Pehuenche, con base en el tamaño de la población, la conectividad y las amenazas.
| Sub-población | Área (m2) | IC | Amenazas | ECE | PC1 | ||||
| Ruta | Sequía/cárcavas | Ganado | Muertes indet. | Salmónidos | |||||
| I. Nacientes | 4,044 | 8.41 | 0 | 0 | 2 | 0 | 1 | 0.88 | Baja |
| II. del Límite | 5,744 | 8.39 | 2 | 3 | 2 | 2 | 3 | -1.26 | Muy alta |
| III. Pichintur | 5,002 | 9.91 | 0 | 0 | 2 | 3 | 2 | 0.08 | Media |
| IV. Rial Rojas | 6,208 | 6.05 | 1 | 3 | 2 | 1 | 2 | -0.73 | Alta |
| V. Nueva | 2,160 | 9.61 | 1 | 0 | 2 | 3 | 0 | -0.19 | Media |
| VI. Campanaria | 5,860 | 5.26 | 1 | 0 | 1 | 3 | 3 | -0.46 | Media |
| VII. Cajón Largo | 9,550 | 6.64 | 0 | 0 | 1 | 0 | 0 | 1.69 | Muy baja |
1 PC: Prioridad de conservación calculada de acuerdo con Velasco (2018).
Las comparaciones entre las 3 temporadas no mostraron diferencias significativas para ninguno de los grupos estudiados adultos y juveniles/área (H = 1.9935, df = 2, p = 0.369), adultos y juveniles/hora (H = 1.3599, df = 2, p = 0.5066), larvas/área (H = 0.13936, df = 2, p = 0.933) y larvas por hora (H = 0.6051, df = 2, p = 0.7389). Entre meses, la diferencia no fue significativa para larvas (H = 4.5278, df = 5, p = 0.4762 por área, H = 4.0986, df = 5, p = 0.5353 por hora), en cambio para los postmetamórficos solo fue significativa para los conteos por área (H = 13.97, df = 5, p = 0.0158). Los análisis post hoc indican menores cantidades de adultos y juveniles en febrero respecto a diciembre (p = 0.0075) y enero (p = 0.0021), como también en marzo respecto a enero (p = 0.0185, fig. 2).
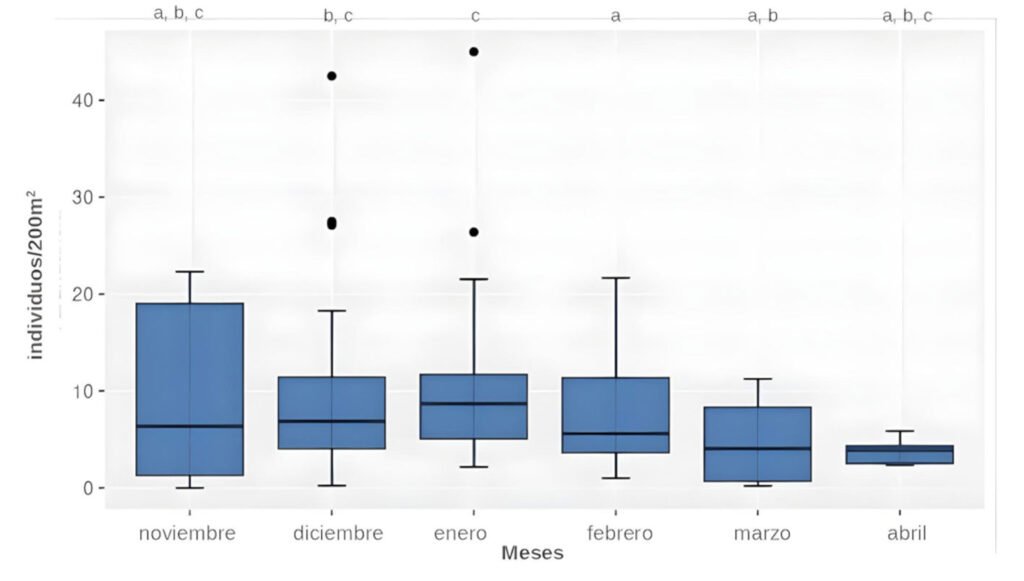
Figura 2. Variación mensual de la abundancia de adultos y juveniles (individuos/200 m2) de Alsodes pehuenche. Los meses con diferentes códigos de letras indican diferencias significativas (p < 0.05).
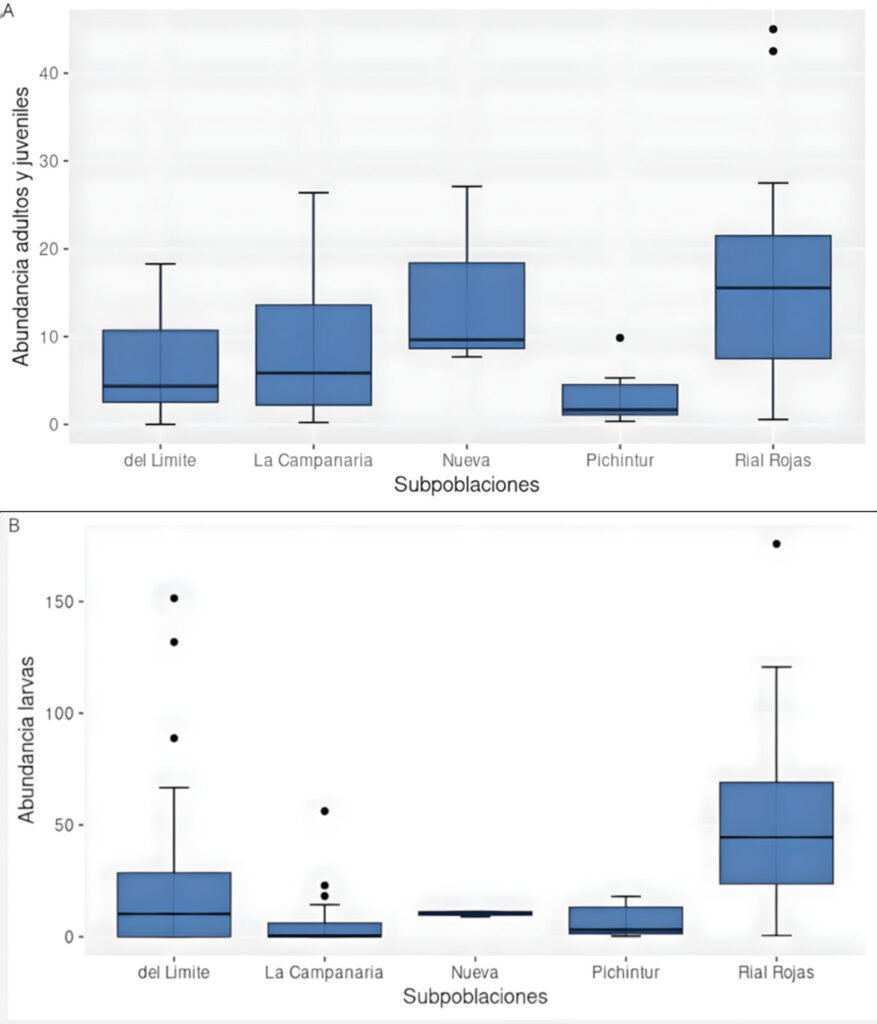
Figura 3. Abundancia de Alsodes pehuenche (individuos/200 m2) entre 5 subpoblaciones. A, Adultos y juveniles; B, larvas. Las subpoblaciones con diferentes códigos de letras indican diferencias significativas (p < 0.05).
De los 14 arroyos del valle Pehuenche con presencia de A. pehuenche, los arroyos A tienen un área de ocupación de 49% respecto de los arroyos B con 51%. Y en los 12 arroyos donde se realizaron conteos por área, éstos fueron mayores en los arroyos A que en los B, tanto para adultos y juveniles (H = 31.977, df = 2, p < 0.001), como para larvas (H = 10.42, df = 2, p = 0.0055).
Se reconocieron 7 subpoblaciones en el valle Pehuenche: Nacientes, del Límite, Pichintur, Rial Rojas, Nueva, Campanaria y Cajón Largo (fig. 1). De éstas, se tienen datos de conteo de 5 (todas, excepto Nacientes y Cajón Largo). Cuando se comparan estas 5 subpoblaciones, se encuentran diferencias significativas tanto para adultos y juveniles/área (H = 17.436, df = 4, p = 0.0016), como para larvas/área (por área H = 23.639, df = 4, p < 0.001). Se registraron más adultos y juveniles en las subpoblaciones Rial Rojas y Nueva, y más larvas en Rial Rojas (fig. 3). Sin embargo, estas diferencias no son significativas cuando se analizan los datos de postmetamórficos/hora (H = 8.8053, df = 5, p = 0.1171) y larvas/hora (H = 4.0986, df = 5, p = 0.5353).
El único lugar donde se encontraron solo larvas fue en una charca de la subpoblación del Límite. Éstas coexistían con larvas y adultos de rana de 4 ojos (Pleurodema bufoninum). Además, esta última especie ha sido observada eventualmente coexistiendo con A. pehuenche en las subpoblaciones Rial Rojas, Campanaria y del Límite. Otras especies registradas durante los muestreos, que se identifican como posibles depredadores de A. pehuenche son el zorro gris (Lycalopex gymnocercus), el chiñe o zorrino (Conepatus chinga), la gaviota capucho café (Chroicocephalus maculipennis) y el águila mora (Geranoaetus melanoleucus).
Aunque la detección de huevos no fue objeto de este estudio, dado que no se realizó búsqueda activa en oquedades ni se manipularon hembras, en noviembre 2022 se observó una masa de 20 huevos aproximadamente, flotando en una pequeña poza de la subpoblación Rial Rojas en época de deshielo. Los huevos no eran pigmentados y cada uno midió entre 6 y 7 mm de diámetro.
Los índices de conectividad calculados para cada una de las subpoblaciones se muestran en la tabla 3. El arroyo Pehuenche representa la principal conexión entre subpoblaciones. En este arroyo se encontraron individuos adultos en la desembocadura de arroyos de primer orden pertenecientes a las subpoblaciones del Límite, Rial Rojas, Nueva y Campanaria (tabla 1). Si bien Cajón Largo tiene relativamente bajo índice de conectividad, el área de esta subpoblación es la mayor de todas.
Se registraron 24 individuos muertos durante el periodo de estudio: 1) 6 individuos adultos —2 hembras, 2 machos y 2 adultos indeterminados— en el mes de enero de la temporada estival 2021 y 2 en la temporada 2022-2023 en los muestreos realizados en la ruta internacional ARG145; 2) 3 individuos —1 adulto indeterminado y 2 larvas— encontrados muertos por desecación sobre el curso de un arroyo de Rial Rojas que mostró una disminución abrupta del caudal luego del deshielo entre noviembre y diciembre 2022; 3) 4 adultos —3 machos y 1 indeterminado— encontrados con signos de depredación (patas traseras lastimadas o mutiladas) al costado o dentro del curso del arroyo donde se registra presencia de salmónidos, esto es, en las subpoblaciones del Límite y Campanaria; 4) 6 adultos —2 machos y 4 indeterminados—, 1 juvenil indeterminado y 2 larvas encontrados muertos sin causa evidente, dentro del curso del arroyo o próximo a éste.
Finalmente, los valores de ECE negativos usados como indicadores, permitieron identificar a las subpoblaciones del Límite y Rial Rojas como unidades de conservación prioritarias, de las cuales la del Límite requiere esfuerzos más urgentes.
Discusión
Corbalán et al. (2010) reportaron 355 individuos de A. pehuenche en 2,500 m lineales (equivalente a 14 individuos/200 m2) en un muestreo realizado en 2008 en arroyos de la subpoblación del Límite. Si se compara ese valor con el obtenido en este estudio para los mismos arroyos en el mismo mes en 2021 (8 individuos/200 m2), el primero es mayor, pero se encuentra dentro del intervalo de variación de este estudio. Cabe mencionar que se usaron 2 técnicas diferentes para el conteo (búsqueda activa de individuos en oquedades durante el día y encuentro visual nocturno, con 3 observadores en los 2 casos), por lo que, con la información disponible actualmente, solo la mortalidad de individuos reportada desde 2008 permite estimar una tendencia poblacional decreciente para esta subpoblación.
En 2 arroyos muy cercanos a la subpoblación del Límite en Chile, Correa et al. (2013) reportaron 20 y 24 adultos/h o 15 y 66 adultos/200 m². Estos últimos datos corresponden a muestreos nocturnos realizados en marzo de 2012, con 4 observadores. La cantidad máxima de adultos por área observados por Correa et al. (2013) es mayor al máximo obtenido en este estudio, lo cual puede deberse a un error de muestreo en solo 30 m lineales, o bien a fluctuaciones poblacionales naturales (Kissel et al., 2020). En arroyos de Rial Moreno, a 21 km al NE de la población del valle Pehuenche, Corbalán et al. (2023) reportaron 26 adultos/hora y 4 juveniles/hora en un muestreo nocturno realizado en enero de 2019, los cuales se encuentran dentro de valores obtenidos en el valle Pehuenche en este estudio.
Durante las 3 temporadas consecutivas muestreadas en este trabajo, no se detectaron cambios significativos para ningún estadio de desarrollo de A. pehuenche. Esto puede deberse a que se trata de un periodo corto en términos de monitoreo. Por lo tanto, es necesario mantener los muestreos para conocer tendencias poblacionales a largo plazo. Por otro lado, la detección de individuos por encuentro visual a lo largo de los arroyos, podría compararse con otros estudios enfocados al monitoreo poblacional de la especie para lograr establecer un óptimo en términos de esfuerzo de muestreo. Dado que estas investigaciones dependen del presupuesto disponible y de limitaciones logísticas (Joseph et al., 2006) principalmente ligadas a la elevada demanda de horas/persona en un ambiente de alta montaña, se debe buscar una estrategia que permita satisfacer tanto la demanda de datos pertinentes para la gestión, como la obtención de información ecológica de calidad (Stephens et al., 2015; Yoccoz et al., 2001).
En relación con la primera acción de conservación llevada a cabo en la población del valle Pehuenche, que consistió en la construcción de 800 m de cordón o bordillo en la ruta y 2 cámaras de infiltración para evitar que la sal vertida en la ruta llegue a los arroyos, provocó la deshidratación y muerte de 18 individuos que fueron encontrados en diciembre de 2017, y 50 en enero de 2018. Posiblemente, la construcción a posteriori de rampas entre el cordón o bordillo y la calzada en febrero de 2018, ha evitado que se vuelvan a registrar mortalidades tan abruptas. Si bien nunca pudo constatarse el uso de las rampas por parte de la especie, las mismas habrían mejorado la conexión entre las secciones superior e inferior de los arroyos. Esto demuestra la necesidad de seguimiento de las acciones de conservación que se llevan a cabo. Pero, por otro lado, si bien la ruta pudo haber provocado una disminución poblacional (Corbalán et al., 2010; IUCN, 2019), el número elevado de individuos muertos registrado en este estudio alerta sobre la necesidad de avanzar en el conocimiento de otras amenazas, como la depredación por salmónidos invasores, el cambio climático y la infección por el hongo quitridio (Batrachochytrium dendrobatidis) de los anfibios.
En este trabajo, de todos los estadios evaluados, las larvas fueron las más abundantes, las más estables en el tiempo y presentaron el mismo patrón de variación que los adultos entre arroyos A y B, y subpoblaciones (fig. 3). Al igual que lo reportado por Corbalán et al. (2010, 2023), se encontraron larvas de diferentes tamaños conviviendo en pozas, pero también se observaron en rápidos, remansos y debajo de la vegetación acuática. Estos resultados son coherentes con la estrategia de especies con desarrollo larval plurianual (Úbeda, 2021). Por otro lado, la menor cantidad de juveniles registrada respecto de adultos podría ser un problema de detectabilidad por su tamaño (Petrovana y Schmidt, 2019), podría corresponder a una segregación por microhábitats según el estadio (Gonwouo et al., 2022), o bien, ser evidencia de una dinámica poblacional particular solo en esta fase de su ciclo de vida (Kissel et al., 2020).
Los muestreos preliminares realizados de día y de noche demuestran que hay arroyos permanentes que solo tienen adultos. Los estudios a futuro podrán determinar las causas que posiblemente puedan estar asociadas con aspectos fisicoquímicos del agua, o a la dispersión de individuos. La información actual permite suponer que las rutas de dispersión de A. pehuenche con base en sus hábitos acuáticos, son los mismos arroyos. Los registros de individuos secos en la ruta, principalmente entre diciembre y enero, al igual que la mayor cantidad de adultos y juveniles registrados en estos meses (fig. 2), podrían estar indicando una mayor dispersión en esa temporada del año.
Los arroyos del valle Pehuenche son los mejores conocidos hasta el momento y el área que ocupan representa solo 6% del área de distribución de A. pehuenche (487.9 km²) reportada por Corbalán et al. (2023). De la misma manera, habiendo considerado en nuestro estudio casi todos los arroyos presentes en el valle Pehuenche, representa 0.8% del área de ocupación total para la especie (4.84 km²; Corbalán et al., 2023). El espacio sin ocupación efectiva de la especie, actualmente, es muy grande (Corbalán et al., 2023), aunque en el pasado pudo haber estado presente en un área mayor si se comparan los registros de presencia en Cajón Grande (Cei y Roig, 1965; Corbalán et al., 2023). Si se considera desde la descripción de la especie en 1965, A. pehuenche ha reducido su área de distribución histórica, lo cual puede deberse a diferentes causas. La depredación por salmónidos en la actualidad podría ser la causa de que la especie haya quedado restringida solo a las zonas altas de las cuencas. Por ello, es necesario conocer la distribución de salmónidos e identificar dónde coexisten estos peces y la rana. Donde esto ocurre en el valle Pehuenche, se registraron adultos muertos de A. pehuenche con signos de depredación. Así, los salmónidos representan una barrera para la dispersión de las ranas, reduciendo la conectividad entre subpoblaciones y dentro de ellas entre arroyos A y B (tabla 3). Como se ha demostrado para otras especies de anfibios, la consecuencia es la disminución del intercambio genético o demográfico (e.g., Kacoliris et al., 2022; Velasco, 2018). Por lo tanto, para conservar las subpoblaciones se requiere asegurar su conectividad, tanto entre las secciones inferior y superior de los arroyos A impactados por la ruta, como dentro de las subpoblaciones entre los arroyos A y B, y entre las subpoblaciones conectadas a través del arroyo Pehuenche.
Las observaciones de campo sugieren que los individuos se distribuyen de manera agrupada a lo largo de los cursos de agua y vegas, no solo las larvas en las pozas, sino también los adultos a lo largo de los arroyos. Esto debe ser evaluado en la etapa reproductiva a inicios de la temporada y al final de ésta, cuando el caudal disminuye y algunos cauces se secan total o parcialmente, donde solo quedan pozas pequeñas con agua. Si este efecto es acentuado por la disminución de cobertura permanente de nieve en la cuenca del Río Grande (Aumassanne et al., 2019), es posible que más arroyos, que todavía mantienen agua hasta el final de la temporada, se sequen como ha ocurrido en los arroyos A3 y A4 de la población del Límite, aumentando así la fragmentación del hábitat. Si A4 no se hubiera secado, las 3 subpoblaciones (del Límite, Pichintur y Rial Rojas) estarían conectadas, formando una sola subpoblación (fig. 1).
Si bien gran parte de las amenazas han sido identificadas (Corbalán et al., 2010, 2023), la mayoría no han sido cuantificadas. Con base en las observaciones realizadas durante los muestreos en este estudio, se sistematizaron las amenazas para cada arroyo y cada subpoblación en el valle Pehuenche (tablas 2, 3). Como resultado de la priorización, las subpoblaciones del Límite y Rial Rojas se identifican como prioritarias para iniciar acciones concretas de conservación. Es posible que ambas sean fragmentos de una población mayor y que hayan estado unidas cuando el arroyo intermedio no estaba seco. En la del Límite se podría iniciar la mitigación de la depredación por salmónidos y reducir los efectos de la ruta. Es necesario continuar con el seguimiento y cuantificación de las amenazas, considerar las aún no evaluadas, tales como las que pueden estar generando las actividades turísticas, el vertido de sal en la ruta, crecidas extraordinarias y otras potenciales como el tendido de líneas de alta tensión, planificada desde el Maule (Chile) al río Diamante (San Rafael, Argentina).
El avance logrado en 20 años de estudios sobre la biología y conservación de este anfibio endémico de Argentina y Chile nos brinda herramientas para sostener acciones concretas de manejo que alivien alguna de las amenazas y que aseguren la viabilidad de las poblaciones a largo plazo. Se espera que un programa de monitoreo anual permita avanzar en el conocimiento de las tendencias poblacionales a largo plazo. Las subpoblaciones del valle Pehuenche son las mejor conocidas hasta el momento y han sufrido disminuciones, fragmentaciones y también extinciones locales. La priorización de su estado de conservación brinda herramientas para implementar acciones necesarias a corto plazo.
Agradecimientos
Por la colaboración en el trabajo de campo, se agradece a estudiantes de la Tecnicatura en Conservación de la Naturaleza Sede Malargüe del IEF Núm. 9-016, especialmente a Julián Rodríguez, Armando Barros, Francisco Jofré y Pablo Lucero; también a estudiantes voluntarios y Graciela Ríos. A Karen Olate por la elaboración del mapa. Y a un revisor anónimo por sus aportes a la primera versión del manuscrito y a los dos revisores que realizaron aportes importantes para mejorar el manuscrito. La investigación se logró gracias al financiamiento del proyecto SIIP 06/M003 Resol. 3978/2022 UNCUYO y aportes personales de investigadoras y estudiantes.
Referencias
Aumassanne, C. M., Beget, M. E., Di Bella, C. M., Oricchio, P. y Gaspari, F. J. (2019). Cobertura de nieve en las cuencas de los ríos Grande y Barrancas (Argentina) y su relación con la morfometría. Revista de Investigación Agropecuaria, 45, 394–403.
Cei, J. M. (1976). Remarks on some neotropical amphibians of the genus Alsodes from southern Argentina. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano, 117, 159–164.
Cei, J. M. (1980). Amphibians of Argentina. Monitore Zoologico Italiano, N.S. Monografia 2. Florencia: Universitá degli studi di Firenze.
Cei, J. M. y Roig, V. G. (1965). The systematic status and biology of Telmatobius montanus Lataste (Amphibia: Leptodactylidae). Copeia, 4, 421–425.
Corbalán, V., Debandi, G. y Martínez, F. (2010). Alsodes pehuenche (Anura: Cycloramphidae): past, present and future. Cuadernos de Herpetología, 24, 17–23.
Corbalán, V., Debandi, G., Martínez, F. y Úbeda, C. (2014). Prolonged larval development in the critically endangered Pehuenche’s Frog Alsodes pehuenche: implications for conservation. Amphibia–Reptilia, 35, 283–292. http://dx.doi.
org/10.1163/15685381-00002951
Corbalán V., Debandi G., Literas S., Álvarez L., Rivera J. A., Dopazo J. et al. (2023). Newly discovered sites and potential threats for the critically endangered frog, Alsodes pehuenche, in Southern South America. Herpetological Conservation and Biology, 18, 48–56.
Correa, C., Pastenes, L., Iturra, P., Calderón, P., Vásquez, D., Lam, N. et al. (2013). Confirmation of the presence of Alsodes pehuenche Cei, 1976 (Anura, Alsodidae) in Chile: morphological, chromosomal and molecular evidence. Gayana, 77, 117–123. http://dx.doi.org/10.4067/S0717-65382013000200006
Correa, C., Zepeda, P., Lagos, N., Salinas, H., Palma, R. E. y Vásquez, D. (2018). New populations of two threatened species of Alsodes (Anura, Alsodidae) reveal the scarce biogeographic knowledge of the genus in the Andes of central Chile. Zoosystematics and Evolution, 94, 349–358.
Correa, C. J. Morales, C. Schussler y J. C. Ortiz. (2020). An enigmatic population of Alsodes (Anura, Alsodidae) from the Andes of central Chile with three species-level mitochondrial lineages. Mitochondrial DNA Part A DNA Mapping, Sequencing, and Analysis, 31, 25–34. https://doi.org/10.1080/24701394.2019.1704744
Crump, M. L. y Scott, N. J. (1994). Visual encounter surveys. En W. R.Heyer, M. A. Donnelly, R. W. Mc Diarmid, L. A. C. Hayek y M. S. Foster (Eds.). Measuring and Monitoring Biological Diversity Standard Methods for Amphibians (pp. 84–92). Washington D.C.: Smithsonian Institution Press.
Cuevas, C. C. y Formas, J. R. (2003). Cytogenetic analysis of four species of the genus Alsodes (Anura: Leptodactylidae) with comments about the karyological evolution of the genus. Hereditas, 138, 138–147. https://doi.org/10.1034/j.1601-5223.2003.01677.x
Garreaud, R., Vuille, M., Compagnucci, R. y Marengo, J. (2009). Present-day south american climate. Palaeogeography, Palaeoclimatology, Palaeoecology, 281, 180–195. http://dx.
doi.org/10.1016/j.palaeo.2007.10.032
Ghirardi, R., Levy, M. G., López, J. A., Corbalán, V., Steciow, M. M. y Perotti, M. G. (2014). Endangered amphibians infected with the chytrid fungus Batrachochytrium dendrobatidis in austral temperate wetlands from Argentina. Herpetological Journal, 24, 129–133.
Gonwouo, N. L. Schäfer, M. y Tsekané, S. J. (2022). Goliath frog (Conraua goliath) abundance in relation to frog age, habitat, and human activity. Amphibian and Reptile Conservation, 16, e319.
Grant, E. H. C., Miller, D. A. y Muths, E. (2020). A synthesis of evidence of drivers of amphibian declines. Herpetologica, 76, 101–107. https://doi.org/10.1655/0018-0831-76.2.101
Green D. M., Lannoo M. J., Lesbarrères D. y Muths E. (2020). Amphibian population declines: 30 years of progress in confronting a complex problem. Herpetologica, 76, 97–100. https://doi.org/10.1655/0018-0831-76.2.97
Herrera, F. y Velásquez, N. A. (2016a). Dimorfismo sexual en Alsodes pehuenche Cei 1976 (Amphibia, Anura, Alsodidae). Boletín Chileno de Herpetología, 3, 4–6.
Herrera, F. y Velásquez, N. A. (2016b). Uso de cuevas en Alsodes pehuenche Cei 1976 (Amphibia, Anura, Alsodidae). Boletín Chileno de Herpetología, 3, 17–20.
Jensen, I. S., Haugen, V. F. y Skålin, R. (2007). Yr. Norwegian Meteorological Institute and the Norwegian Broadcasting Corporation. Recuperado el 15 de agosto, 2024 de https://www.yr.no/nb
Joseph, L. N., Field, S. A., Wilcox, C. y Possingham, H. P. (2006). Presence-absence versus abundance data for monitoring threatened species. Conservation Biology, 20, 1679–1687. https://doi.org/10.1111/j.1523-1739.2006.00529.x
Kacoliris, F. P., Berkunsky, I., Acosta, J. C., Acosta, R., Agostini, M. G., Akmentins, M. et al. (2022). Current threats faced by amphibian populations in the southern cone of South America. Journal for Nature Conservation, 69, 126254. https://doi.org/10.1016/j.jnc.2022.126254
Kissel, A. M., Tenan, S. y Muths, E. (2020). Density dependence and adult survival drive dynamics in two high elevation amphibian populations. Diversity, 12, 478.
Lin, J. P. (2009). The functional linkage index: a metric for measuring connectivity among habitat patches using least-cost distances. Journal of Conservation Planning, 5, 28–37.
Llano, C., Durán, V., Gasco, A., Reynals, E. y Zárate, M. S. (2021). Traditional puesteros´ perceptions of biodiversity in semi-arid Southern Mendoza, Argentina. Journal of Arid Environments, 192, 104553. https://doi.org/10.1016/j.ja
ridenv.2021.104553
Luedtke, J. A., Chanson, J., Neam, K., Hobin L., Maciel, A. O., Catenazzi, A. et al. (2023). Ongoing declines for the world’s amphibians in the face of emerging threats. Nature, 622, 308–314. https://doi.org/10.1038/s41586-023-06235-w
Luja, V., Rodríguez-Estrella, H. R., Schaub, M. y Schmidt, B. R. (2015). Among-population variation in monthly and annual survival of the Baja California treefrog, Pseudacris hypochondriaca curta, in desert oases of Baja California Sur, Mexico. Herpetological Conservation and Biology, 10, 112–122.
Pereyra, L. C., Etchepare, E. y Vaira, M. (2021). Manual de técnicas y protocolos para el relevamiento y estudio de anfibios de Argentina. San Salvador de Jujuy: Editorial de la Universidad Nacional de Jujuy.
Petrovana, S. O. y Schmidt, B. R. (2019). Neglected juveniles; a call for integrating all amphibian life stages in assessments of mitigation success (and how to do it). Biological Conservation, 236, 252–260. https://doi.org/10.1016/j.biocon.
2019.04.016
Piñeiro, A., Fibla, P., López, C., Velásquez, N. y Pastenes, L. (2020). Characterization of an Alsodes pehuenche breeding site in the Andes of central Chile. Herpetozoa, 33, 21–26. https://doi.org/10.3897/herpetozoa.33.e49268
Pollock, J. E. (2006). Detecting population declines over large areas with presence-absence, time-to-encounter, and count survey methods. Conservation Biology, 20, 882–892. https://doi.org/10.1111/j.1523-1739.2006.00559.x
Prado, W. S., Meriggi, J., Martínez, F. y Corbalán, V. (2019). Ampliación del área de distribución de Alsodes pehuenche en Argentina. Revista del Museo de La Plata, 4 (Suplem.), 1R–117R.
Rivera, J. A., Marianetti, G. y Hinrichs, S. (2018). Validation of CHIRPS precipitation dataset along the Central Andes of Argentina. Atmospheric Research, 213, 437–449. https:
//doi.org/10.1016/j.pce.2022.103184
Stephens, P. A., Pettorelli, N., Barlow, J., Whittingham, M. J. y Cadotte, M. W. (2015). Management by proxy? The use of indices in applied ecology. Journal of Applied Ecology, 52, 1–6. https://doi.org/10.1111/1365-2664.12405
Úbeda, C. (2021). Estrategias reproductivas, hábitats y otros aspectos ecológicos de los anfibios altoandinos en la vertiente oriental de la Cordillera de los Andes. Boletín Chileno de Herpetología, 8, 10–21.
UICN (Unión Internacional para la Conservación de la Naturaleza), Species Survival Commission Amphibian Specialist Group. (2019). Alsodes pehuenche. IUCN Red List of threatened species, 2019. https://www.iucnredlist.org
Vaira, M., Akmentins, M., Attademo, M., Baldo, D., Barrasso, D., Barrionuevo, S. et al. (2012). Categorización del estado de conservación de los anfibios de la República Argentina. Cuadernos de Herpetología, 26 (Suplem. 1), 131–159.
Vaira, M., Pereyra, L. C., Akmentins M. S. y Bielby, J. (2017). Conservation status of Amphibians of Argentina: An update and evaluation of national assessments. Amphibian y Reptile Conservation, 11, e135.
Velasco, M. A. (2018). Dinámica poblacional y conservación de la ranita del Valcheta (Pleurodema somuncurense) (Cei, 1969), Patagonia, Argentina (Tesis doctoral). Universidad Nacional de La Plata, La Plata, Buenos Aires.
Velasco, M. A., Kacoliris, F. P., Berkunsky, I., Quiroga, S. y Williams, J. D. (2016). Current distributional status of the critically endangered Valcheta frog: implications for conservation. Neotropical Biology and Conservation, 11, 110–113. http://dx.doi.org/10.4013/nbc.2016.112.08
Vidal, M. A., Henríquez, N., Torres-Díaz, C., Collado, G. y Acuña-Rodríguez, I. S. (2024). Identifying strategies for effective biodiversity preservation and species status of Chilean amphibians. Biology, 13, 169. https://doi.org/10.33
90/biology13030169
Yoccoz, N. G., Nichols, J. D. y Boulinier, T. (2001). Monitoring of biological diversity in space and time. Trends in Ecology and Evolution, 16, 446–453. https://doi.org/10.1016/S0169-
5347(01)02205-4
Zarco, A., Corbalán, V. y Debandi, G. (2020). Depredación por truchas arcoíris invasoras en la rana de pecho espinoso Pehuenche, en peligro crítico. Journal of Fish Biology, 98, 878-880. https://doi.org/10.1111/jfb.14401
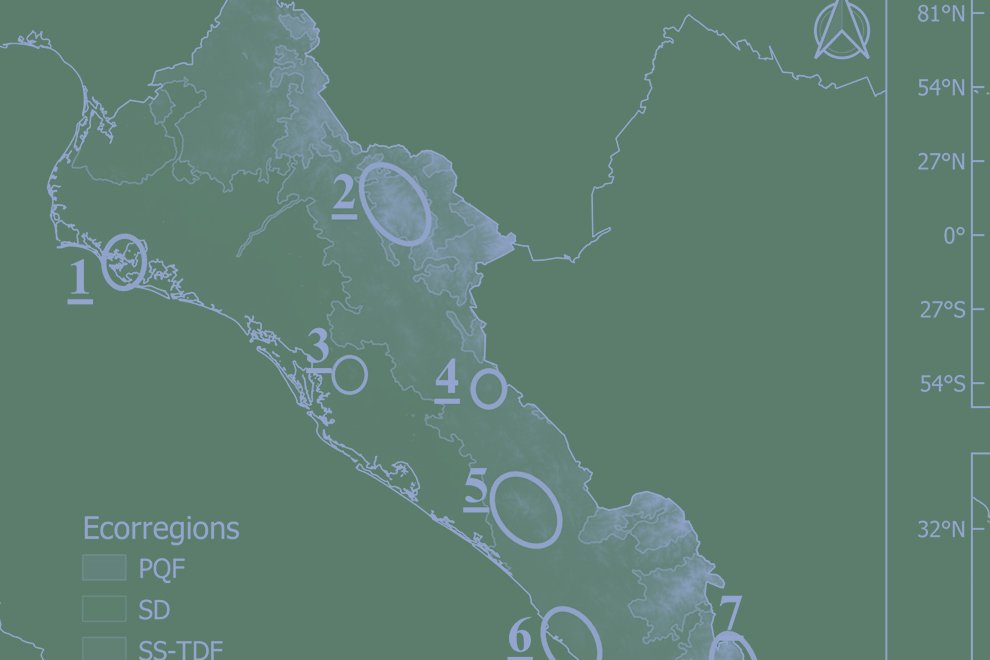
Priority areas for conservation based on endemic vascular plant species and their biocultural attributes: a case study in Sinaloa, Mexico
C. Rocío Álamo-Herrera a, María Clara Arteaga a, *, Rafael Bello-Bedoy b
a Instituto Politécnico Nacional, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Unidad Durango, Sigma No. 119, Fracc. 20 de Noviembre II, 34234 Victoria de Durango, Durango, Mexico
b Universidad de Guadalajara, Centro Universitario de Ciencias Biológicas y Agropecuarias, Cátedras Conahcyt-Universidad de Guadalajara, Camino Ramón Padilla Sánchez No. 2100, 45200 Zapopan, Jalisco, Mexico
*Corresponding author: d1j17kk@hotmail.com (J.F. Pío-León)
Received: 20 February 2024; accepted: 02 July 2024
Abstract
Endemic vascular plants are one of the main biodiversity indicators used to propose priority conservation areas. The richness of endemic species and corrected and weighted endemism are the most frequently used criteria, while anthropogenic or biocultural factors such as ethnobotanical value or ecological vulnerability are seldom considered. This work proposes priority conservation areas for Sinaloa, Mexico, considering the richness of its endemic species, corrected and weighted endemism, as well as ethnobotanical value, protection status, and the Priority Conservation Index (PCI). The analysis was performed in a 19 × 19 km grid and included 247 records of 78 species. The areas proposed when considering only the richness of endemic species and the weighted endemism coincided with previously known areas of high biodiversity in the state, which are areas of high collection effort and low anthropogenic impact. When considering the ethnobotanical value and protection status, the areas identified included those with greater anthropogenic impact, which contained species of biocultural and economic importance. When the PCI was used, both of these types of regions were identified. We therefore recommend this index as a better indicator to select priority areas.
Keywords: Conservation index; Ebenopsis caesalpinioides; Ethnobotanical value; Protected Natural Areas; Priority species; Stenocereus martinezii
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Áreas prioritarias para la conservación con base en especies de plantas vasculares endémicas y sus atributos bioculturales: un estudio de caso en Sinaloa, México
Resumen
Las plantas vasculares endémicas son uno de los principales indicadores empleados para proponer áreas prioritarias de conservación. La riqueza de especies endémicas y el endemismo ponderado y corregido son frecuentemente incluidos en los análisis, mientras que aspectos antropogénicos o bioculturales como el valor etnobotánico o la vulnerabilidad ecológica son poco considerados. Este trabajo propone áreas prioritarias de conservación para Sinaloa, México, considerando su riqueza de especies endémicas, endemismo ponderado y corregido, así como el valor etnobotánico, estatus de protección e índice prioritario de conservación (IPC). El análisis se realizó en cuadrículas de 19 × 19 km e incluyó 274 registros de 78 especies. Las áreas resultantes, considerando únicamente la riqueza de especies y el endemismo ponderado, coinciden con áreas previamente conocidas por su alta biodiversidad en el estado, mismas que poseen altos esfuerzos de colectas y bajos impactos antropogénicos. Por el contrario, cuando se consideró el valor etnobotánico y el estatus de protección, las áreas prioritarias incluyen zonas con alto impacto antropogénico, pero con presencia de especies con importancia biocultural y valor económico. Empleando el IPC se identificaron ambos tipos de regiones; en consecuencia, recomendamos este índice como un mejor indicador para seleccionar áreas prioritarias.
Palabras clave: Índice de conservación; Ebenopsis caesalpinioides; Valor etnobotánico; Áreas naturales protegidas; Especies prioritarias; Stenocereus martinezii
Introduction
Plants are essential organisms for maintaining the equilibrium of ecosystems and life on Earth. They provide the vast majority of the ecosystem and subsistence services that humans need to survive, including food, medicine, shelter, oxygen, carbon capture, and soil retention. Caring for plants is therefore an act of self-preservation (Raven, 2018). However, over 50% of the terrestrial vegetation on Earth is severely or moderately altered (Bradshaw et al., 2021).
Mexico is the country with the third to fifth highest plant richness, with more than 23,000 species, half of which are endemic (Conabio, 2023a; Villaseñor & Meave, 2022). However, despite 12% of Mexican territory being decreed as Protected Natural Area, it is estimated that between 37 and 50% of the nation’s land area has been impacted by human activities and that the majority of well-conserved areas are located in desert, semi-desert, and high mountain areas that are difficult to access (González-Abraham et al., 2015; Mora, 2019). Two of the largest and most biodiverse ecosystems in the country —dry forest and temperate forest— have suffered total degradation of 37 and 26% of their cover, respectively (Conabio, 2023b; Ulloa-Ulloa et al., 2017). The main causes of this deforestation have been agriculture and infrastructure development, both in Mexico specifically and worldwide (González-Abraham et al., 2015; Laso-Bayas et al., 2022).
One of the main analytical approaches used to propose priority conservation areas is grid analysis, which identifies centers of high biodiversity (“hotspots”) using criteria such as species richness, richness of endemic species, weighted endemism (WE), presence of threatened species, diversity of specific taxa (families or genera), or phylogenetic richness (Gutiérrez-Rodríguez et al., 2022; Maassoumi & Ashouri, 2022; Mehta et al., 2023; Murillo-Pérez et al., 2022; Qin et al., 2022; Sosa & De-Nova, 2012; Vargas-Amado et al., 2020; Villaseñor et al., 2022). The richness of endemic species in particular has the advantage of using a more precise (though smaller) database than the other aforementioned criteria for grid analysis to indicate conservation priority areas.
On the other hand, other indices can be used to propose conservation priority species based on their ethnobotanical or biocultural value, or the degree of threat they face due to use (e.g., Value of Use, Frequency of Use, Conservation Index) (De Lucena et al., 2013; Dhar et al., 2000; Mehta et al., 2023; Pío-León et al., 2023). However, these indices are not usually included in grid richness analyses to select priority conservation areas. These indices weight each species’ value based on its conservation priority, such that a priority conservation area would be determined not just by the total number of species or endemism, but also by their qualities.
Pío-León et al. (2023) compiled a list of the vascular plant species of Sinaloa and proposed some priority conservation areas based on the presence of 2 or more endemic species. In addition, the authors proposed a Priority Conservation Index (PCI) for each species based on its ethnobotanical value and ecological vulnerability, considering characteristics such as its distribution, habitat, and anthropogenic threats. In this index, species with high ethnobotanical value, slow growth (arboreal habit), threatened habitat (near to agricultural zones), and small distribution area (1 or a few known localities), have higher priority than those with no known ethnobotanical value, rapid growth (herbs), inaccessible habitat (cliffs or steep slopes), and wide distribution. The PCI was calculated with the formula:
PCI= D + H + Fv + Am + VE + Vc
where D is distribution; H, habitat; Fv, life form or habit (Spanish abbreviation); Am, degree of threat to their populations; VE, ethnobotanical value, and Vc, commercial value. However, that work did not perform a grid richness analysis to incorporate the values of these indices with traditional algorithms such as WE.
In the present work, we propose priority conservation areas in Sinaloa considering 3 types of algorithms: 1) richness of endemic species, WE, and corrected weighted endemism (CWE); 2) ethnobotanical value, protection status (NOM-059-SEMARNAT-2010 or IUCN) and PCI, and 3) the combination of 1) and 2). We hypothesized that incorporating those anthropogenic and biocultural attributes would modify the priority conservation areas selected since they will not necessarily correspond to the areas of the highest species richness.
Materials and methods
Sinaloa is located in northwestern Mexico, bordered on the east by the Sierra Madre Occidental (SMO) and on the west by the Pacific Ocean. According to Wiken et al. (2011), the main level III ecoregions that compose it are: 1) Sinaloa and Sonora Hills and Canyons with Xeric Shrub and Low Tropical Deciduous Forest (SS-TDF) (50%, 27,568 km2), which is located in the low parts of the SMO; 2) Sinaloa Coastal Plain with Low Tropical Thorn Forest and Wetland (S-TF) (29%, 15,612 km2), located in the lowlands near the coast, from the south-central portion northward; and 3) SMO with Conifer, Oak, and Mixed Forests (PQF) (15.78%, 8,681 km2) in the high parts of the western slope of the SMO (Fig. 1). It has been estimated that 4,000 species of vascular plants, nearly 80 of them endemic, occur in Sinaloa (Pío-León et al., 2023; Vega-Aviña et al., 2021). However, a large part of the coastal territory has been converted to agricultural land (~ 28,000 km2) (INEGI, 2023), resulting in severely fragmented habitats.
The database was based on the file generated by Pío-León et al. (2023) with some updates (Table 1). We incorporated recently described species (until October 2023) and removed species and collections that lacked reliable geographic coordinates. In addition, we prepared a matrix of weighted values considering the ethnobotanical value (E; 1 = documented use, 0 = no documented use), inclusion in a risk category (R) by the NOM-059-SEMARNAT-2010 (Semarnat, 2019) or IUCN (2023) (1 = included in at least 1 category, 0 = not included), and the value of the Conservation Priority Index (PCI), using the values reported by Pío-León et al. (2023) (Table 1). For the PCI, we assigned values according to their quartile position: 4 (upper quartile), 3 (second quartile), 2 (third quartile), and 1 (lower quartile). From these data, we formed 3 analysis groups: 1) biocultural value (E+R; 0 to 2), 2) PCI value (1 to 4), and 3) PCI + R (1 to 5).
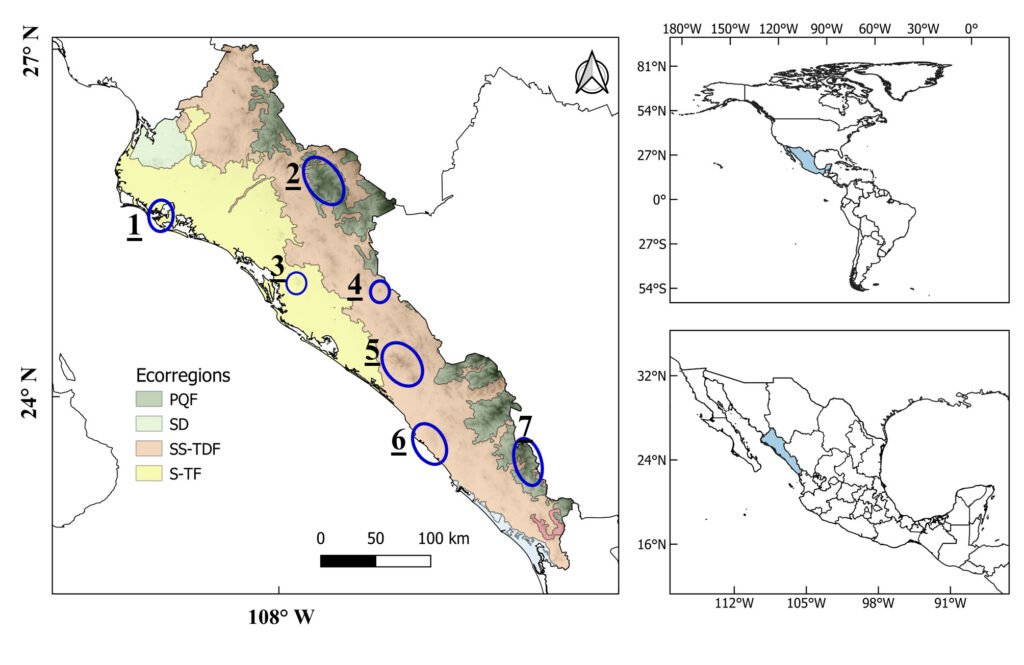
Figure 1. Sinaloa state, Mexico, its main ecoregions level III (Wiken et al., 2011), and the regions of endemism according to Pío-León et al. (2023). Ecoregions: PQF = Conifer, Oak, and Mixed Forests of the Sierra Madre Occidental; SD = Sonoran Desert; SS-TDF = Sinaloa and Sonora Hills and Canyons with Xeric Shrub and Low Tropical Deciduous Forest; S-TF = Sinaloa Coastal Plain with Low Tropical Thorn Forest and Wetlands. Regions of endemism: 1 = Maviri-Topolobampo, 2 = Surutato region, 3 = Cerro Tecomate, 4 = Cerro Colorado, 5 = Sierra Tacuichamona, 6 = Meseta de Cacaxtla, 7 = Sierra de Concordia.
Table 1
List of endemic species of Sinaloa considered for this study and their scores by attributes. E = Ethnobotanical value; R = species with conservation status by the NOM-059-SEMARNAT-2010 or the IUCN (risk); PCI = Priority Conservation Index according to their quartile position.
| Especies | E | R | PCI | E+R | PCI+R |
| Acourtia gentryi L. Cabrera | 0 | 0 | 2 | 0 | 2 |
| Acourtia sinaloana B.L. Turner | 0 | 0 | 1 | 0 | 1 |
| Ageratina concordiana B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Albizia ortegae Britton & Rose | 0 | 0 | 3 | 0 | 3 |
| Aloysia nahuire A.H. Gentry & Moldenke | 1 | 0 | 4 | 1 | 5 |
| Anemia brandegeei Davenp. | 0 | 0 | 1 | 0 | 1 |
| Arachnothryx sinaloae Borhidi | 0 | 0 | 2 | 0 | 2 |
| Bastardiastrum tarasoides Fryxell | 0 | 0 | 2 | 0 | 2 |
| Bastardiastrum wissaduloides (Baker f.) Bates | 0 | 0 | 1 | 0 | 1 |
| Bletia santosii H. Ávila, J.G. González & Art. Castro | 0 | 0 | 2 | 0 | 2 |
| Bourreria franciscoi Pío-León & Vega | 0 | 0 | 3 | 0 | 3 |
| Bourreria ritovegana Pio-León, M.G. Chávez & L.O. Alvarado | 0 | 0 | 3 | 0 | 3 |
| Bouvardia sinaloae Borhidi & E. Martínez | 0 | 0 | 2 | 0 | 2 |
| Calliandra estebanensis H.M. Hern. | 0 | 0 | 2 | 0 | 2 |
| Carlowrightia fuertensis T.F. Daniel | 0 | 0 | 3 | 0 | 3 |
| Castilleja racemosa (Breedlove & Heckard) T.I. Chuang & Heckard | 0 | 0 | 3 | 0 | 3 |
| Chrysactinia lehtoae D.J. Keil | 0 | 0 | 2 | 0 | 2 |
| Cnidoscolus sinaloensis Breckon ex Fern.Casas | 0 | 1 | 3 | 1 | 4 |
| Cochemiea thomasii García-Mor., Rodr. González, J. García-Jim. & Iamonico | 0 | 0 | 2 | 0 | 2 |
| Coutaportla helgae Pío-León, Torr.-Montúfar & H. Ávila | 0 | 0 | 1 | 0 | 1 |
| Coutaportla lorenceana Torr.-Montúfar, H. Ochot. & Art.Castro | 0 | 0 | 2 | 0 | 2 |
| Croton ortegae Standl. | 0 | 0 | 3 | 0 | 3 |
| Ctenodon rosei Morton | 0 | 0 | 3 | 0 | 3 |
| Cuphea delicatula Brandegee | 0 | 0 | 2 | 0 | 2 |
| Cyclanthera monticola Gentry | 0 | 0 | 2 | 0 | 2 |
| Dioscorea sinaloensis O. Téllez | 0 | 0 | 2 | 0 | 2 |
| Dryopetalon breedlovei (Rollins) Al-Shehbaz | 0 | 0 | 1 | 0 | 1 |
| Ebenopsis caesalpinioides (Standl.) Britton & Rose | 1 | 1 | 4 | 2 | 5 |
| Echeveria coppii Moran ex Gideon F.Sm. & Bischofberger | 0 | 0 | 2 | 0 | 2 |
| Echeveria juliana Reyes, González-Zorzano & Kristen | 0 | 0 | 1 | 0 | 1 |
| Echeveria kimnachii J. Meyrán & R. Vega | 0 | 0 | 1 | 0 | 1 |
| Epidendrum petacaense Hágsater, J. Duarte & Pío-León | 0 | 0 | 2 | 0 | 2 |
| Eryngiophyllum rosei Greenm. | 0 | 0 | 2 | 0 | 2 |
| Frangula surotatensis (Gentry) A. Pool | 0 | 0 | 2 | 0 | 2 |
| Graptopetalum sinaloensis Vega | 0 | 0 | 1 | 0 | 1 |
| Guardiola stenodonta S.F. Blake | 0 | 0 | 1 | 0 | 1 |
| Helicteres vegae Cristóbal | 0 | 0 | 3 | 0 | 3 |
| Heliopsis sinaloensis B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Table 1. Continued | |||||
| Especies | E | R | PCI | E+R | PCI+R |
| Hofmeisteria sinaloensis Gentry | 0 | 0 | 1 | 0 | 1 |
| Indigofera sinaloensis M. Sousa & Cruz Durán | 0 | 0 | 2 | 0 | 2 |
| Ipomopsis monticola J.M. Porter & L.A. Johnson | 0 | 0 | 2 | 0 | 2 |
| Iresine arenaria Standl. | 0 | 0 | 1 | 0 | 1 |
| Koanophyllum concordianum B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Lasianthaea gentryi B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Lasianthaea ritovegana B.L. Turner | 0 | 0 | 1 | 0 | 1 |
| Licania mexicana Lundell | 0 | 0 | 2 | 0 | 2 |
| Lobelia macrocentron (Benth.) T.J. Ayers | 0 | 0 | 2 | 0 | 2 |
| Lopezia conjugens Brandegee | 0 | 0 | 1 | 0 | 1 |
| Lopezia sinaloensis Munz | 0 | 0 | 1 | 0 | 1 |
| Lupinus gentryanus C.P. Sm. | 0 | 0 | 2 | 0 | 2 |
| Lupinus howard-scottii C.P. Sm. | 0 | 0 | 2 | 0 | 2 |
| Lupinus sinaloensis C.P. Sm. | 0 | 0 | 2 | 0 | 2 |
| Mariosousa gentryi Seigler & Ebinger | 0 | 0 | 3 | 0 | 3 |
| Mimosa coelocarpa B.L. Rob. | 0 | 0 | 3 | 0 | 3 |
| Mitracarpus aristatus Borhidi & Lozada-Pérez | 0 | 0 | 2 | 0 | 2 |
| Molinadendron sinaloense (Standl. & Gentry) P.K. Endress | 0 | 1 | 3 | 1 | 4 |
| Pavonia gentryi Fryxell | 0 | 0 | 2 | 0 | 2 |
| Peniocereus papillosus (Britton & Rose) U. Guzmán | 0 | 0 | 2 | 0 | 2 |
| Periptera trichostemon Bullock | 0 | 0 | 2 | 0 | 2 |
| Perityle canescens Everly | 0 | 0 | 1 | 0 | 1 |
| Perityle grandifolia Brandegee | 0 | 0 | 1 | 0 | 1 |
| Perityle stevensii B.L. Turner | 0 | 0 | 1 | 0 | 1 |
| Physalis vestita Waterf. | 0 | 0 | 3 | 0 | 3 |
| Pitcairnia monticola Brandegee | 0 | 0 | 1 | 0 | 1 |
| Polygala polyedra Brandegee | 0 | 0 | 1 | 0 | 1 |
| Psacalium quercifolium H.Rob. & Brettell | 0 | 0 | 2 | 0 | 2 |
| Salvia beltraniorum J.G.González, Pío-León & Art.Castro | 0 | 0 | 2 | 0 | 2 |
| Salvia trichostephana Epling | 0 | 0 | 2 | 0 | 2 |
| Sedum copalense Kimnach | 0 | 0 | 1 | 0 | 1 |
| Stenocereus martinezii (J.G. Ortega) Bravo | 1 | 1 | 4 | 2 | 5 |
| Stevia concordiana B.L. Turner | 0 | 0 | 2 | 0 | 2 |
| Sysyrinchium jacquelineanum Art.Castro, H. Ávila & J.G. González | 0 | 0 | 1 | 0 | 1 |
| Tibouchina thulia Todzia | 0 | 0 | 1 | 0 | 1 |
| Tillandsia mazatlanensis Rauh | 0 | 0 | 2 | 0 | 2 |
| Tillandsia occulta H. Luther | 0 | 0 | 2 | 0 | 2 |
| Verbesina microcarpa S.F. Blake | 0 | 0 | 2 | 0 | 2 |
| Verbesina ortegae S.F. Blake | 0 | 0 | 2 | 0 | 2 |
| Verbesina sinaloensis B.L. Turner | 0 | 0 | 2 | 0 | 2 |
Richness of endemism (SR), weighted endemism (WE), and corrected weighted endemism (CWE). The richness of endemic species was quantified in 19 × 19 km cells (361 km2), dividing Sinaloa into 195 cells. The cell size used was determined according to the criterion of Oyala (2020). Endemic species richness was quantified as the total number of endemic species whose distribution includes the cell. Endemism was evaluated using the WE and CWE indices. The WE score for each cell was obtained by summing, for each species present in the cell, the inverse of the number of cells in which the species occurs; thus, a high WE value indicates cells that contain more species with restricted distributions (i.e., that are found in few other cells), while low WE values indicate cells that mostly contain widely distributed species (i.e., species that are also present in other cells). The CWE is similar, but additionally corrects for potential biases due to differences in overall richness by dividing the value of the WE by the number of species present in the cell (Laffan & Crisp, 2003). The 3 parameters (SR, WE, and CWE) were estimated in the program Biodiverse v.2.0 (Laffan et al., 2010). Geoprocessing of the data was performed in QGIS 3.4.8 (QGIS.org, 2019).
Endemism weighted by biocultural attributes and PCI. In addition to SR, WE, and CWE analysis, endemism weighted by biocultural attributes was evaluated using 2 sets of attribute/parameter combinations, each resulting in 3 maps, 9 in total (Fig. 2). The first set included the species richness plus the biocultural values, resulting in the following 3 combinations: species richness plus biocultural value (SR+E+R), species richness plus PCI (SR+PCI), and PCI plus the risk category (SR+PCI+R). The second set did not consider species richness, resulting in the combinations of biocultural value (E+R), PCI, and PCI+R. For this second set of analyses, only species that fulfilled the relevant criteria were included (e.g., the E+R combination included only species that had ethnobotanical value and are included in a risk category). As such, in the first set of maps, a priority conservation area depended by the number of species present and their qualities (e.g., species with ethnobotanical value or species with protected status), while in the second only the species’ qualities were considered.
The final priority conservation areas were based on the consensus map of the 9 different endemism maps. The consensus areas took into account only the cells that had the highest possible value of the relevant variables in at least 1 of the 9 previously generated endemism maps. The consensus values were obtained by summing the number of times each cell had the highest possible value in each of the endemism maps, such that the highest possible consensus value was theoretically 9 (the cell had the highest possible value in all maps), and the minimum value was 1 (maximal value in only 1 map). The consensus map was also overlayed with Protected Natural Areas and Priority Terrestrial Regions, land use, and bioclimatic corridors.
Results
Occurrence, conservation (risk) status, and ethnobotanical uses of the endemic species of Sinaloa. The database contained 247 records of 78 species, 30 families, and 61 genera. For 48 of the genera (78.7%), only 1 species of the genus was present. The majority of the records were distributed in the central to the southern region of the state, near the coast, in the Meseta de Cacaxtla Natural Protected Area and the area between the former and Sierra de Tacuichamona, as well as in the Concordia and Surutato mountains of the SMO (Fig. 3; regions 6, 5, and 2, in Figure 1). The 2 level III ecoregions best represented were SS-TDF (166 records/ 40 species) and the PQF (53/ 37), followed by the S-TF (17/ 8) (Fig. 3a). Sixty-nine percent of the records fell outside of the polygons of Protected Natural Areas or Priority Terrestrial Conservation Regions (Fig. 3B). Sixty-eight percent of the species (53) were known from a single locality (either a single collection or collections from locations that are very close to each other).
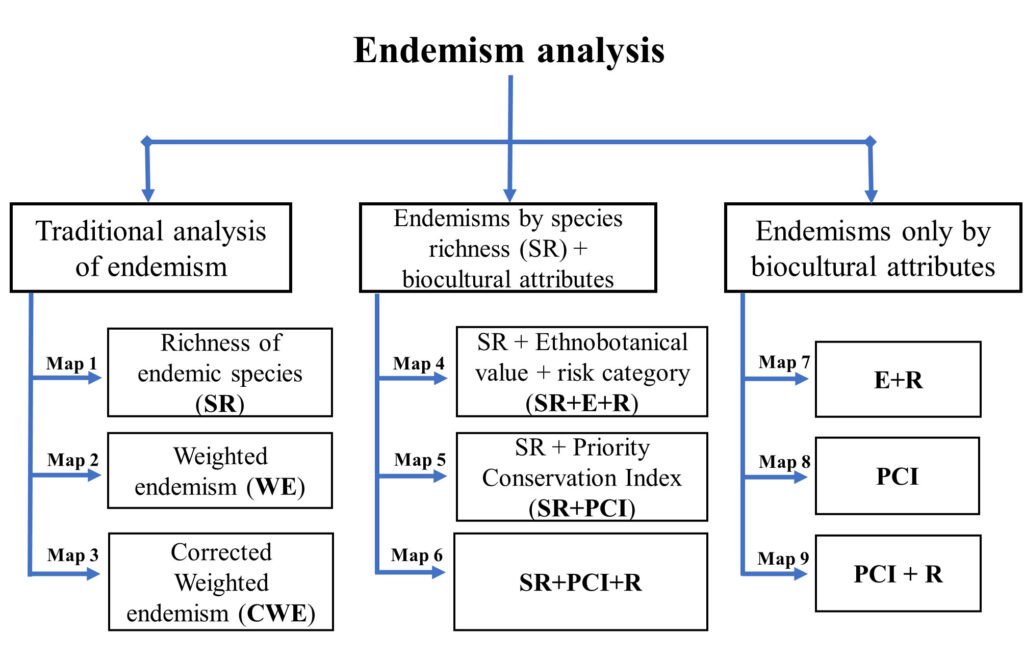
Figure 2. Flowchart of the endemism analysis to select priority areas in Sinaloa, Mexico.
Only 4 species (Cnidoscolus sinaloensis, Ebenopsis caesalpinioides, Molinadendron sinaloense, and Stenocereus martinezii) of the 78 analyzed are found in some risk category (Table 1). All 4 are considered endangered (EN) by the IUCN, while only Stenocereus martinezii is included in NOM-059-SEMARNAT-2010, under the category of special protection (Pr). Only 3 species have well-documented ethnobotanical uses: Aloysia nahuire (aromatic and medicinal tea), Ebenopsis caesalpinioides (edible seeds, occasional commercial value), and Stenocereus martinezii (edible fruits, commercial value). One additional species, Lupinus gentryianus, was noted in the type collection to be used as an anti-parasitic for livestock; however, this plant is only known from that locality, and this use has not since been confirmed, so it was not considered.
The different patterns of endemism are shown in Figure 4. The overall richness of endemism (Fig. 4A) showed 2 main areas —1 in the northern part of the Sierra de Concordia (region 7, Fig. 1) and the other in the western part of the Sierra de Tacuichamona (region 5, Fig. 1)— as well as 3 secondary areas located in the Sierra de Surutato (region 2, Fig. 1), Cerro Colorado (region 4, Fig. 1), and the southern part of the Sierra de Concordia. The WE (Fig. 4B) showed a similar pattern in richness but with an increase in the priority levels of the Sierra de Surutato and a decrease by 1 level for the Tacuichamona and Cerro Colorado. The CWE (Fig. 4C) showed several priority areas more scattered across the state than the WE, mainly in the SMO, corresponding to the majority of the species known from a single locality; however, compared with WE, there was a greater concentration of high-priority cells toward the northern part of the state, near southern Sonora, in the area around the Sierra de Barobampo and Hills of Topolobampo (region 1, Fig. 1).

Figure 3. Records of endemic species in Sinaloa overlayed onto: level III ecoregions (Wiken et al., 2011; definitions in Figure 1) (A) and Protected Natural Areas (PNA) and Priority Terrestrial Regions (B). Categories of Protected Natural Areas: PNAS = state; PNAM = municipal; PNAF = federal; PTR = Priority Terrestrial Regions.
Figure 4. Endemism areas of vascular plants in Sinaloa, Mexico, according to the calculated index values (A-I): SR = endemic species richness; WE = weighted endemism; CWE = corrected weighted endemism; E = ethnobotanical value; R = species with protection status; PCI = Priority Conservation Index.
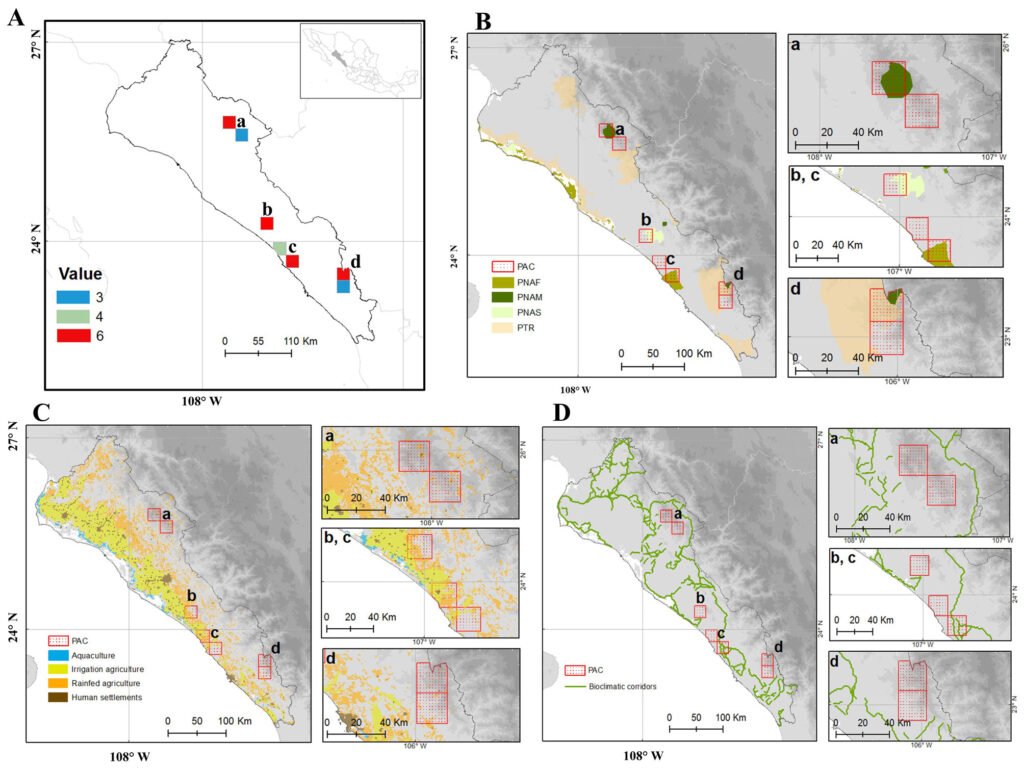
Figure 5. Consensus priority conservation areas (PAC) in the state of Sinaloa (A-D). Consensus map (A) superimposed to: Protected Natural Areas/Priority Terrestrial Regions (B), land use (C), and bioclimatic corridors (D). Categories of Protected Natural Areas: PNAS = state; PNAM = municipal; PNAF = federal; PTR = Priority Terrestrial Regions.
The addition of the ethnobotanical attributes to the protection status and richness of endemic species (SR+E+R) (Fig. 4D) showed an increase in the values for the areas from the Meseta de Cacaxtla (region 6, Fig. 1) to Sierra de Tacuichamona, but a decrease in the zones of the SMO. Adding the Priority Conservation Index to the richness (SR+PCI) (Fig. 4E) showed an increase and homogenization of the priority in all of the aforementioned regions, while adding protection status (SR+PCI+R) (Fig. 3F) did not significantly modify the areas of importance.
Finally, when considering only the ethnobotanical value plus the protection status (E+R), without considering species richness (i.e., eliminating the species that did not have those attributes), the zone of highest priority was concentrated nearly exclusively in the southern part of the state, within and adjacent to the Meseta de Cacaxtla (Fig. 3G). When considering PCI only or PCI plus risk category, there was again a homogenization of the high priority for the 2 mountainous areas (Surutato and Concordia), Meseta de Cacaxtla, Tacuichamona, and surrounding areas (Fig. 4H, I).
The priority conservation areas, as defined by the consensus among the 9 maps analyzed, were composed of 7 polygons grouped into 3 categories (Fig. 5): 4 cells with a value of 6 (of the maximum possible score of 9) in the northern part of the Sierra de Concordia, northwestern part of Sierra Surutato, Meseta de Cacaxtla, and Sierra de Tacuichamona; 1 with a value of 4 in the area between the Meseta de Cacaxtla and Tacuichamona; and 2 with a value of 3 in the southern part of the Sierra de Concordia and southeastern part of the Sierra de Surutato (Fig. 5A). However, since the 3 areas with a value of 3 or 4 were contiguous with areas with a value of 6, 4 priority conservation areas were proposed: Sierra de Surutato (Fig. 45-a), Sierra de Tacuichamona (Fig. 5A-b), Meseta de Cacaxtla (Fig. 5A-c), and Sierra de Concordia (Fig. 5A-d).
Superimposing the consensus map with the map of existing Protected Natural Areas (Fig. 5B) showed that these 4 consensus areas fall partially within protected areas: 1 federal (Área de Protección de Flora y Fauna Meseta de Cacaxtla, Fig. 5B-c), 1 state (Sierra de Tacuichamona, Fig. 5B-b), and 2 municipal (Reserva Chara Pinta in the Sierra de Concordia and Reserva de Surutato, Fig. 5B-d, B-a, respectively). The Sierra de Concordia also includes part of the terrestrial priority region Río Presidio. Regarding land use, the 2 consensus areas in the SMO were found in mixed pine-oak forest with low impact of agricultural activity (Fig. 5C-a, C-d), while the other 2, located in the Sinaloa and Sonora Hills and Canyons with Xeric Shrub and Low Tropical Deciduous Forest ecoregion, present moderate to high impact from irrigated and rainfed agriculture (Fig. 5C-b, C-c). When considering biological corridors, only the priority area in the Meseta de Cacaxtla overlapped with a bioclimatic corridor.
Discussion
The analyses of richness of endemic species and WE showed higher conservation priority in areas that were previously identified as having high endemism (Pío-León et al., 2023), low anthropogenic impact from agriculture, and which have also historically been subject to concentrated collection efforts (Sierra de Surutato and Sierra de Concordia) (Ávila-González et al., 2019; Gentry 1946; Vega-Aviña et al., 2021). On the other hand, the regions defined based on CWE reflected a high number of species known from a single locality, which could indicate the presence of small islands of endemism in the state or low collection effort. In contrast, the inclusion of the ethnobotanical criteria and protection status (E+R) shows a different pattern from species richness, concentrating high priority scored in an area of transition between the coastal plain of Sinaloa and the hills of Sinaloa and Sonora, near the coast in the center-south of the state. These regions correspond to the transition and ecotone between low tropical deciduous forest and thorn forest, which are strongly impacted by anthropogenic activities (irrigated and rainfed agriculture), suggesting that the species with the highest ethnobotanical importance and with protected status (IUCN or NOM-053-SEMARNAT-2010) are found near human activities that require stronger conservation attention than those located in the high parts of the SMO, where the threats are less severe.
The priority conservation areas indicated by the consensus map (Fig. 5) include the regions with the highest richness of endemic species plus the areas with the highest number of species with biocultural importance. These consensus areas are practically the same as those that were assigned the highest priority values when considering only the Priority Conservation Index (PCI) for each species; as such, this index was the most robust single indicator for selecting priority conservation areas. This index combines ethnobotanical parameters such as species’ uses and economic value with ecological parameters such as their distribution, habit, and habitat. Thus, it covers a broad range of criteria that are useful for defining priority species or areas for conservation.
All the priority conservation areas defined by the consensus map (Fig. 5) except 1 included part of a Protected Area polygon, although only 1 was under federal jurisdiction (Meseta de Cacaxtla). The only cell that did not overlap with a Protected Natural Area was adjacent to the Meseta de Cacaxtla, and it was the cell with the largest area of agriculture. This area is important because it contains the 2 species with the highest ethnobotanical value (Ebenopsis caesalpinioides and Stenocereus martinezii), which are also found in a risk category according to the IUCN and NOM-053-SEMARNAT-2010. This area therefore urgently requires conservation and restoration activities, especially for E. caesalpinioides, whose distribution is limited to the area surrounding this cell (Pío-León et al., 2023). Specifically, we recommend avoiding the conversion from rainfed agriculture to technified irrigated agricultural activities, since these are generally more aggressive toward native vegetation. This area is also important because it is located at the transition between lowland deciduous forest and thorn forest of Sinaloa, which could reflect high endemism, in addition to potentially serving as part of the bioclimatic corridor connecting the 2 most important terrestrial ANPs in the state, Meseta de Cacaxtla (federal) and Sierra Tacuichamona (state).
In the present study, the incorporation of the species’ biocultural parameters modified the priority areas for conservation compared to the areas selected when considering only the richness of endemic species, weighted endemism, or corrected weighted endemism. Specifically, the richness analysis identified priority areas in the mountainous and high-diversity regions of Sinaloa, while the ethnobotanical and ecological factors incorporated zones near the coast that have higher anthropogenic impact. The Conservation Priority Index identified all of these priority regions; for this reason, we propose it as a complete and robust index for identifying priority conservation areas. At the state level, we recommend that conservation and restoration actions be implemented in the area of transition between the low tropical deciduous forest and thorn forest. This area simultaneously presents the highest impact of anthropogenic activities and harbors the most important Sinaloa endemic species in terms of biocultural value and protection status —the “pitaya de Sinaloa” (Stenocereus martinezii) and the “guampinola” or “frutilla” (Ebenopsis caesalpinioides). This area should be considered a priority for both conservation and restoration, which would not have been identified as a priority if only the richness of endemism or CWE had been analyzed.
Acknowledgements
The first author is grateful to the Consejo Nacional de Humanidades, Ciencia y Tecnología (Conahcyt) for the grant awarded as part of the Estancias Posdoctorales por México program (I1200/320/2022). We also thank Jorge David López Pérez for his suggestions on data analysis, and the two anonymous reviewers for their comments and suggestions that improved our manuscript.
References
Ávila-González, H., González-Gallegos, J. G., López-Enríquez, I. L., Ruacho-González, L., Rubio-Cardoza, J., & Castro-Castro, A. (2019). Inventario de las plantas vasculares y tipos de vegetación del Santuario El Palmito, Sinaloa, México. Botanical Sciences, 97, 789–820. https://doi.org/10.17129/botsci.2356
Bradshaw, C. J. A., Ehrlich, P. R., Beattie, A., Ceballos, G., Crist, E., Diamond, J. et al. (2021). Underestimating the challenges of avoiding a ghastly future. Frontiers in Conservation Sciences, 1, 615419. https://doi.org/10.3389/fcosc.2020.615419
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2023a). México megadiverso. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Ciudad de México. Retrieved 01 December, 2023 from: https://www.biodiversidad.gob.mx/pais/quees
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2023b). Ecosistemas de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Ciudad de México. Retrieved 01 December, 2023 from: https://www.biodiversidad.gob.mx/ecosistemas/ecosismex
Dhar, U., Rawal, R. S., & Upreti, J. (2000). Setting priorities for conservation of medicinal plants ––a case study in the Indian Himalaya. Biological Conservation, 95, 57–65. https://doi.org/10.1016/S0006-3207(00)00010-0
De Lucena, R. F., Lucena, C. M., Araújo, E. L., Alves, Â. G., & Albuquerque, U. P. D. (2013). Conservation priorities of useful plants from different techniques of collection and analysis of ethnobotanical data. Anais da Academia Brasileira de Ciências, 85,169–186. https://doi.org/10.1590/S0001-37652013005000013
Gentry, H. S. (1946). Notes on the vegetation of Sierra Surotato in northern Sinaloa. Bulletin of the Torrey Botanical Club, 73, 451–462. https://doi.org/10.2307/2481592
González-Abraham, C., Ezcurra, E., Garcillán, P. P., Ortega-Rubio, A., Kolb, M., & Bezaury Creel, J. E. (2015). The human footprint in Mexico: physical geography and historical legacies. Plos One, 10, e0121203. https://doi.org/
10.1371/journal.pone.0121203
Gutiérrez-Rodríguez, B. E., Vásquez-Cruz, M., & Sosa, V. (2022). Phylogenetic endemism of the orchids of Megamexico reveals complementary areas for conservation. Plant Diversity, 44, 351–359. https://doi.org/10.1016/j.pld.20
22.03.004
IUCN (International Union for Conservation of Nature). (2023). The IUCN Red List of Threatened Species. Version 2022-2. Retrieved 01 December, 2023 from: https://www.iucnredlist.org
INEGI (Instituto Nacional de Estadística, Geografía e Informática). (2023). Censo agropecuario 2022. Resultados definitivos Sinaloa. Retrieved 25 January, 2024 from: https://www.inegi.org.mx/
Laso-Bayas, J. C., See, L., Georgieva, I., Schepaschenko, D., Danylo, O., Dürauer, M. et al. (2022). Drivers of tropical forest loss between 2008 and 2019. Scientific Data, 9, 1–8. https://doi.org/10.22022/nodes/06-2021.122
Laffan, S. W., & Crisp, M. D (2003) Assessing endemism at multiple spatial scales, with an example from the Australian vascular flora. Journal of Biogeography, 30, 511–520. https://doi.org/10.1046/j.1365-2699.2003.00875.x
Laffan, S.W., Lubarsky, E., & Rosauer, A. F. (2010) Biodiverse, a tool for the spatial analysis of biological and related diversity. Ecography, 33, 643–647. https://doi.org/10.1111/
j.1600-0587.2010.06237.x
Maassoumi, A. A., & Ashouri, P. (2022). The hotspots and conservation gaps of the mega genus Astragalus (Fabaceae) in the Old-World. Biodiversity and Conservation, 31, 2119–2139. https://doi.org/10.1007/s10531-022-02429-2
Mehta, P., Bisht, K., Sekar, K. C., & Tewari, A. (2023). Mapping biodiversity conservation priorities for threatened plants of Indian Himalayan Region. Biodiversity and Conservation, 32, 2263–2299. https://doi.org/10.1007/s10531-023-02604-z
Mora, F. (2019). The use of ecological integrity indicators within the natural capital index framework: The ecological and economic value of the remnant natural capital of México. Journal for Nature Conservation, 47, 72–92. https://doi.org/10.1016/j.jnc.2018.11.007
Murillo-Pérez, G., Rodríguez, A., Sánchez-Carbajal, D., Ruiz-Sánchez, E., Carrillo-Reyes, P., & Munguía-Lino, G. (2022). Spatial distribution of species richness and endemism of Solanum (Solanaceae) in Mexico. Phytotaxa, 558, 147–177. https://doi.org/10.11646/phytotaxa.558.2.1
Oyala, V. (2020). Sistemas de Información Geográfica. Retrie-
ved on December 25th, 2024 from: http://volaya.es/writing
Pío-León, J. F., González-Elizondo, M., Vega-Aviña, R., González-Elizondo, M. S., González-Gallegos, J. G., Salomón-Montijo, B. et al. (2023). Las plantas vasculares endémicas del estado de Sinaloa, México. Botanical Sciences, 101, 243–269. https://doi.org/10.17129/botsci.3076
QGIS Development Team (2019) Geographic Information System, version 3.4.8. QGIS Association. Retrieved 11 December, 2019 from: http://www.qgis.org
Qin, F., Xue, T., Yang, X., Zhang, W., Wu, J., Huang, Y. et al. (2022). Conservation status of threatened land plants in China and priority sites for better conservation targets: distribution patterns and conservation gap analysis. Biodiversity and Conservation, 31, 2063–2082. https://doi.org/10.1007/s10531-022-02414-9
Raven, P. H. (2018). Saving plants, saving ourselves. Plants People Planet, 1, 8–13. https://doi.org/10.1002/ppp3.3
Semarnat (Secretaría de Medio Ambiente y Recursos Naturales). (2019). Norma Oficial Mexicana NOM-059-SEMARNAT-2010. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación, Ciudad de México. Retrieved 01 December, 2023 from: https://www.dof.gob.mx/nota_detalle.php?codigo=5578808&fecha=14/11/2019#gsc.tab=0
Sosa, V., & De-Nova, J. (2012). Endemic angiosperm lineages in Mexico: hotspots for conservation. Acta Botanica Mexicana, 100, 293–315. https://doi.org/10.21829/abm100.2012.38
Ulloa-Ulloa, C., Acevedo-Rodríguez, P., Beck, S., Belgrano, M. J., Bernal, R., Berry, P. E. et al. (2017). An integrated assessment of the vascular plant species of the Americas. Science, 358, 1614–1617. https://doi.org/10.1126/science.aao
0398
Vargas-Amado, G., Castro-Castro, A., Harker, M., Vargas-Amado, M. E., Villaseñor, J. L., Ortiz, E. et al. (2020). Western Mexico is a priority area for the conservation of Cosmos (Coreopsideae, Asteraceae), based on richness and track analysis. Biodiversity and Conservation, 29, 545–569. https://doi.org/10.1007/s10531-019-01898-2
Vega-Aviña, R., Vega-López, I. F., & Delgado-Vargas, F. (2021). Flora nativa y naturalizada de Sinaloa. Culiacán, Sinaloa: Universidad Autónoma de Sinaloa.
Villaseñor, J. L., & Meave, J. A. (2012). Floristics in Mexico today: insights into a better understanding of biodiversity in a megadiverse country. Botanical Sciences, 100, S14–S33. https://doi.org/10.17129/botsci.3050
Villaseñor, J. L., Ortiz, E., & Hernández-Flores, M. M. (2022). The vascular plant species endemic or nearly endemic to Puebla, Mexico. Botanical Sciences, 101, 1207–1221. https://doi.org/10.17129/botsci.3299
Wiken, E., Jiménez-Nava, F., & Griffith, G. (2011). North American Terrestrial Ecoregions-Level III. Montreal, Canada: Commission for Environmental Cooperation. Retrieved Jul 17, 2021 from: http://www3.cec.org/islando
ra/en/item/10415-north-american-terrestrial-ecoregionsle
vel-iii

Notas científicas